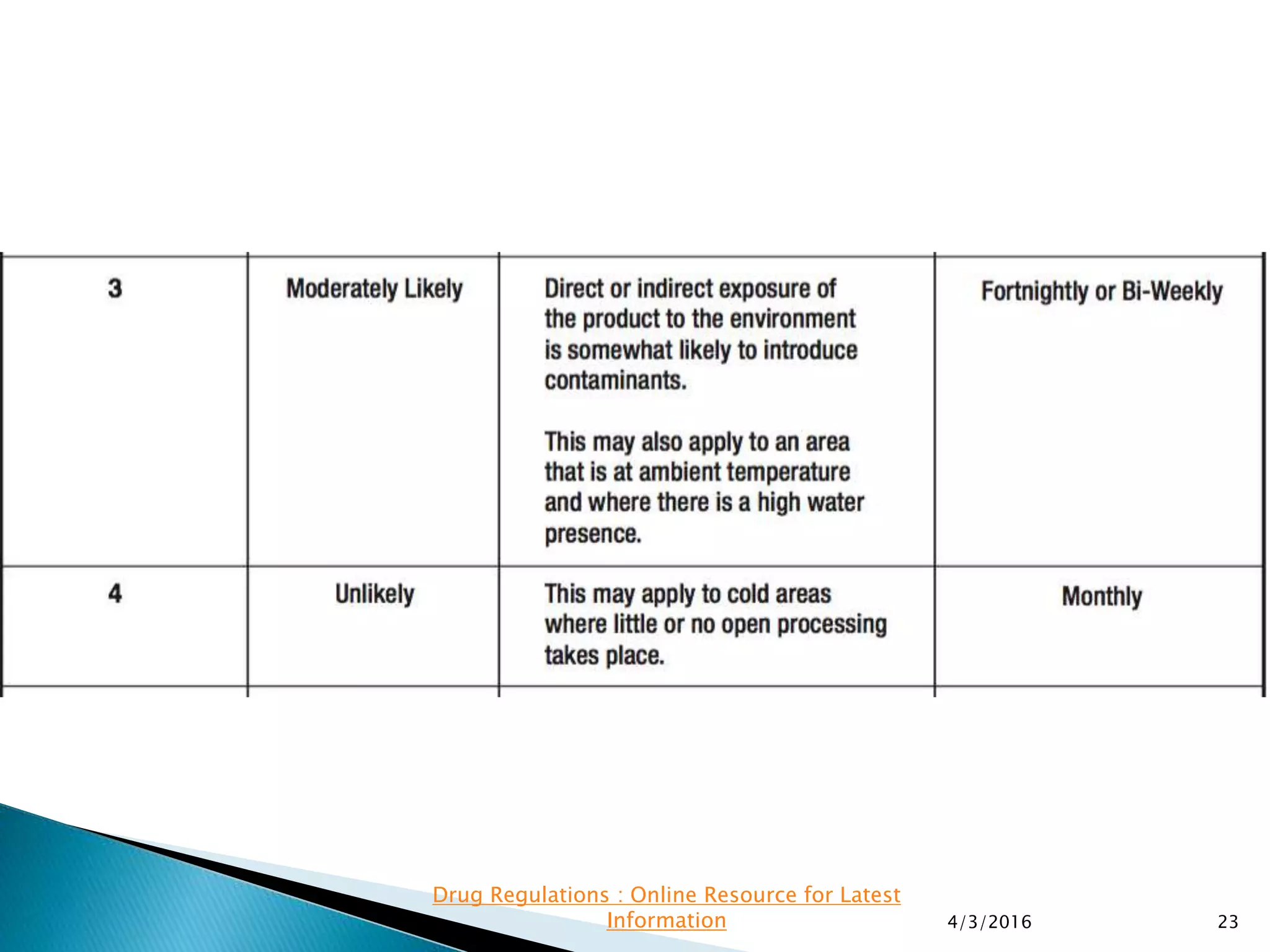
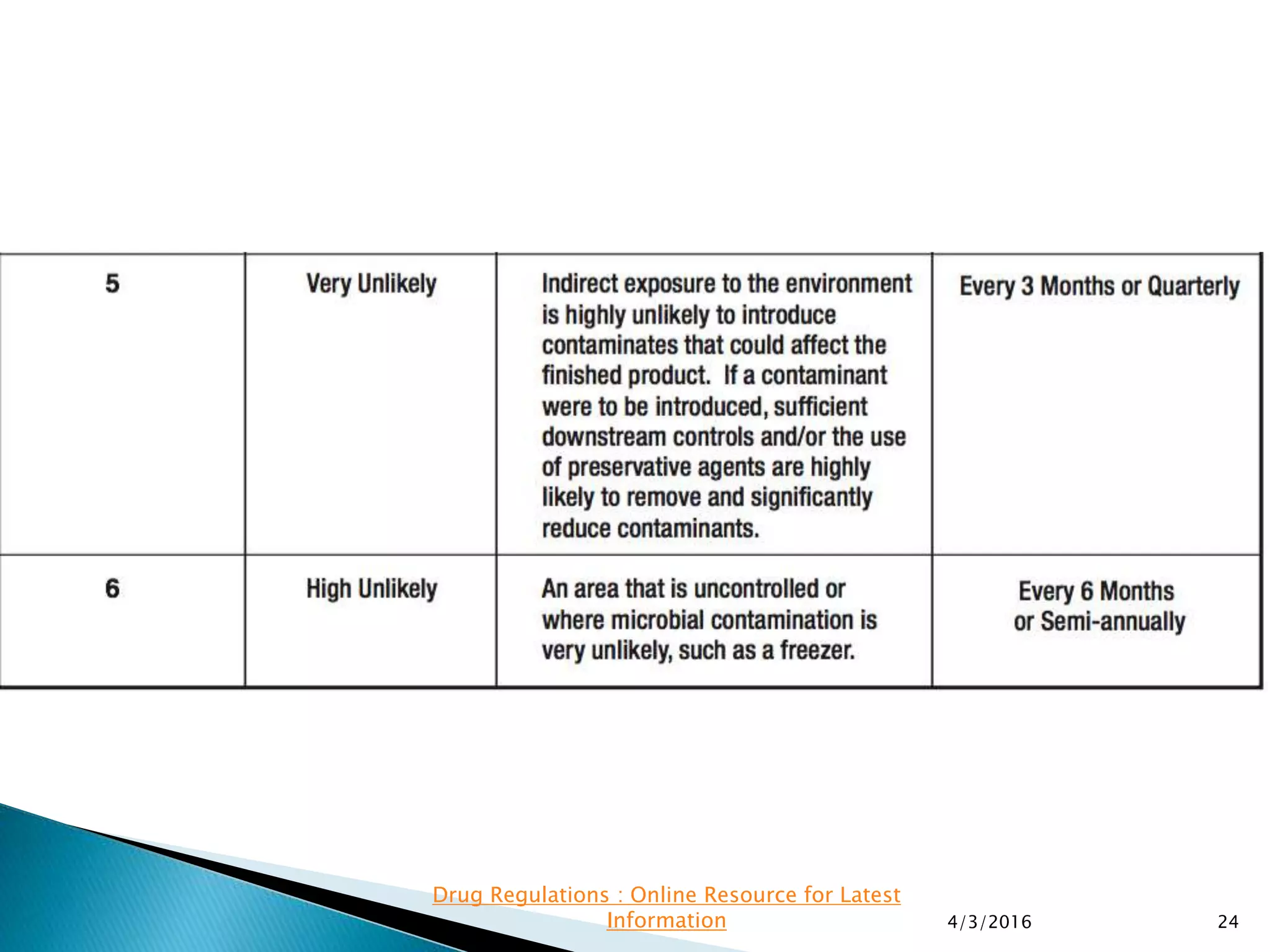
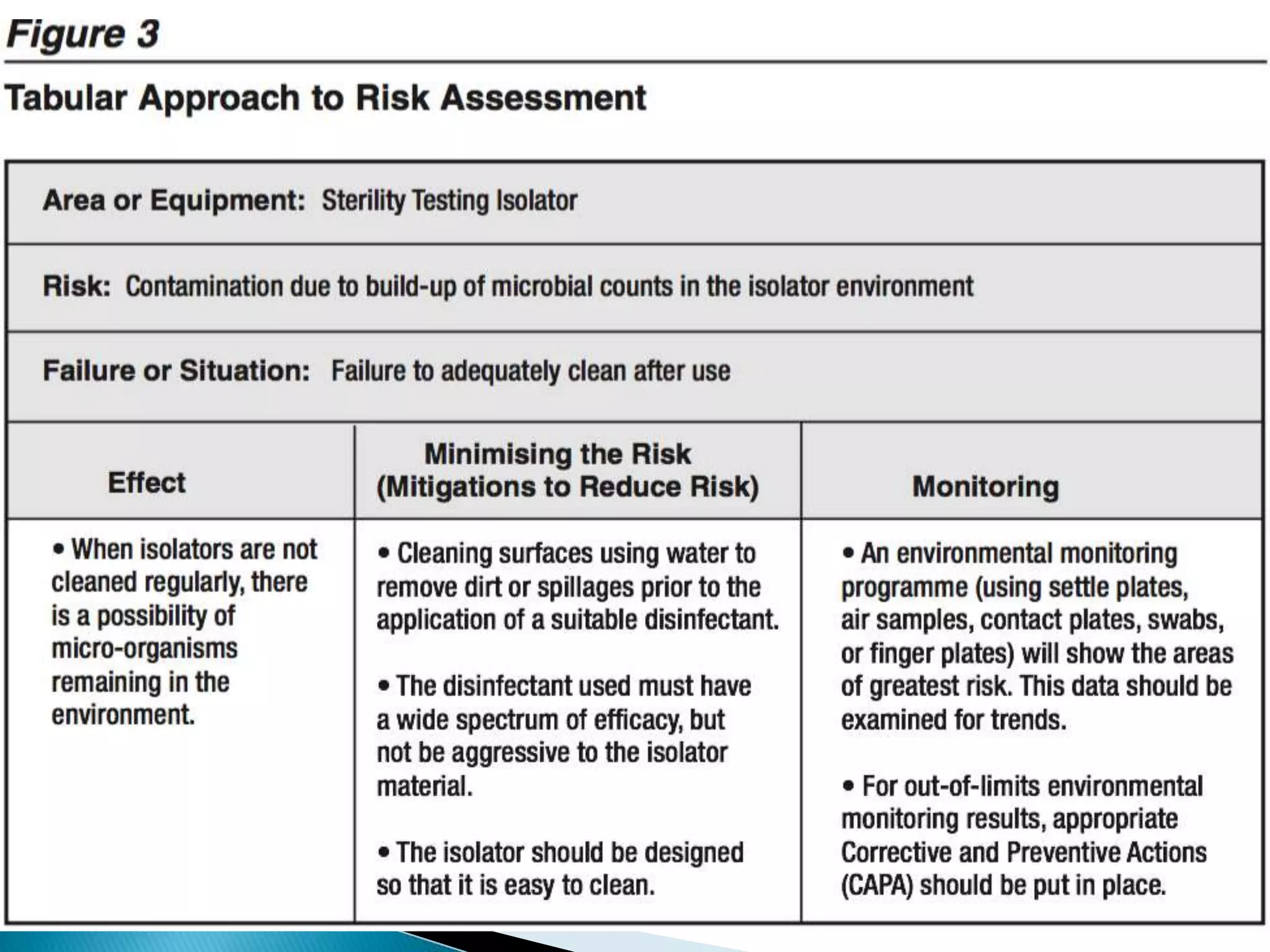
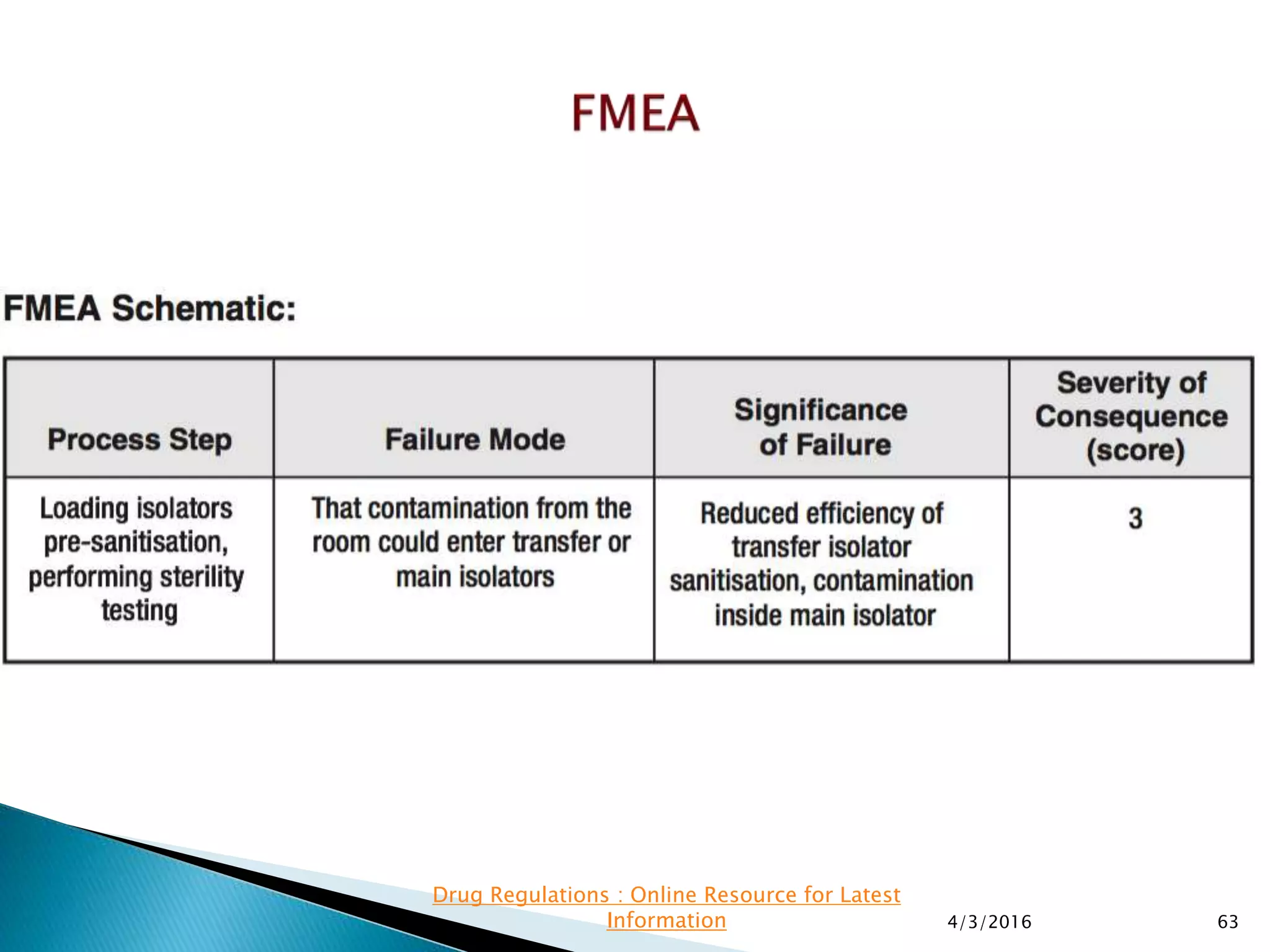
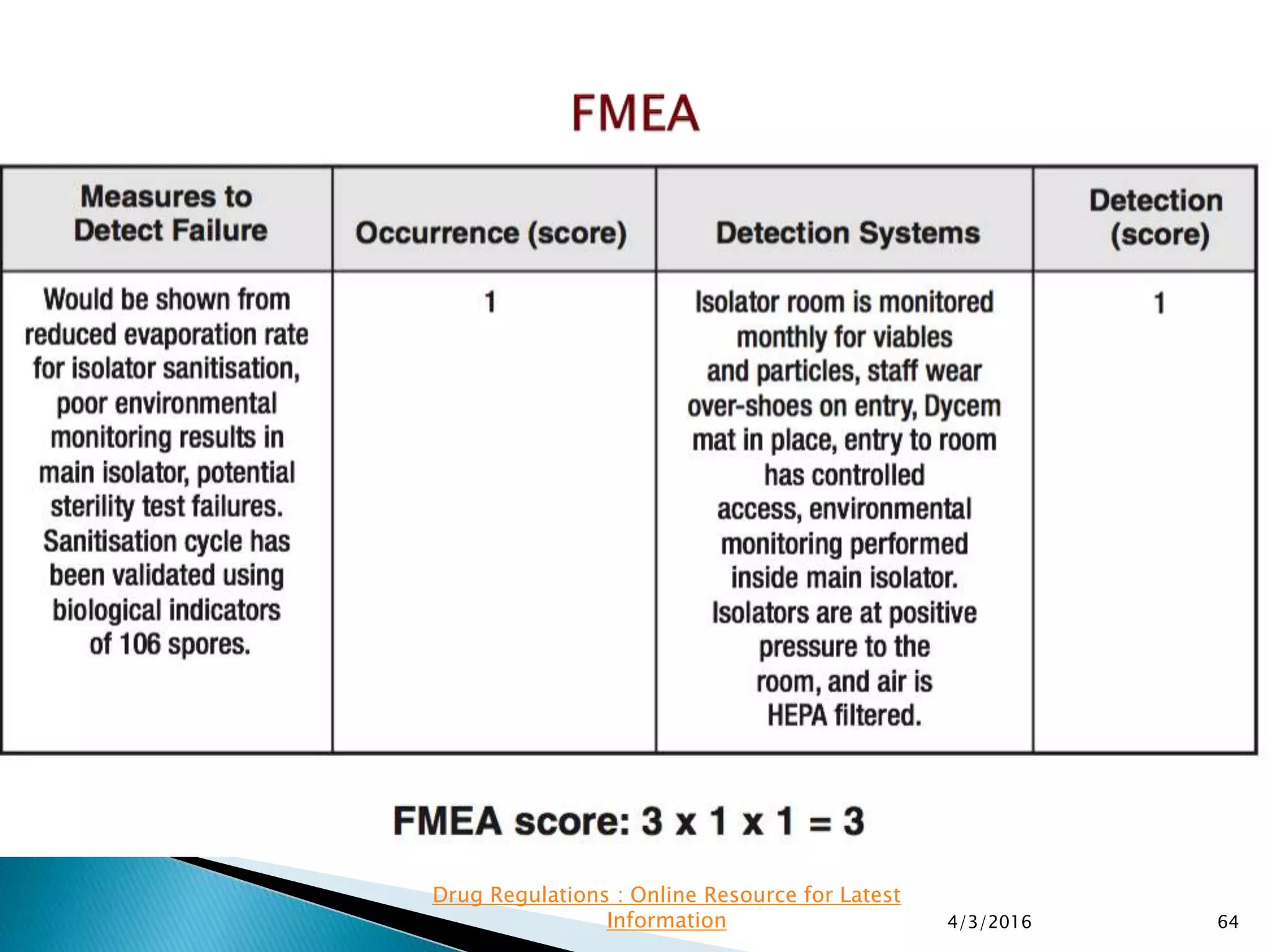
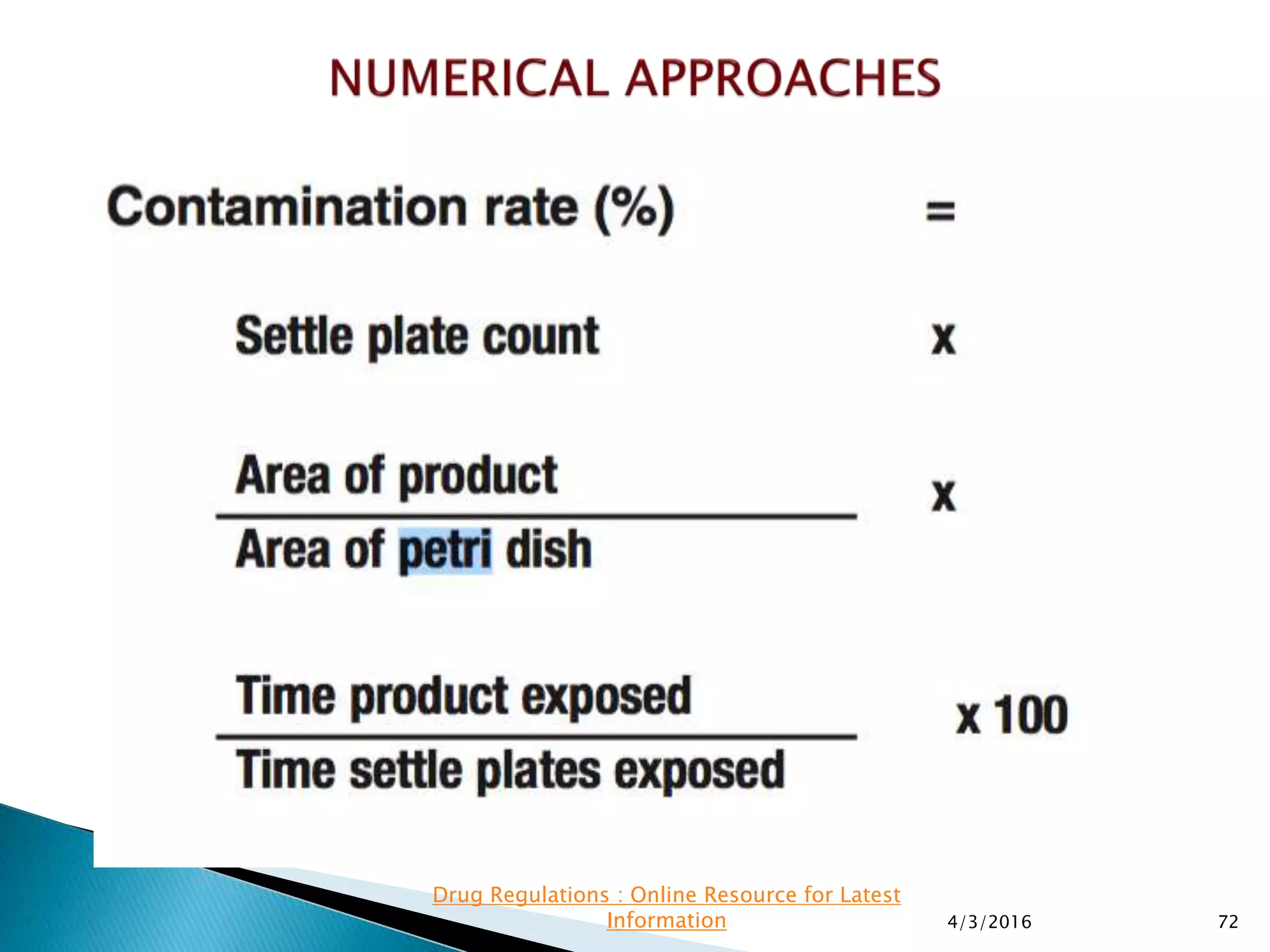
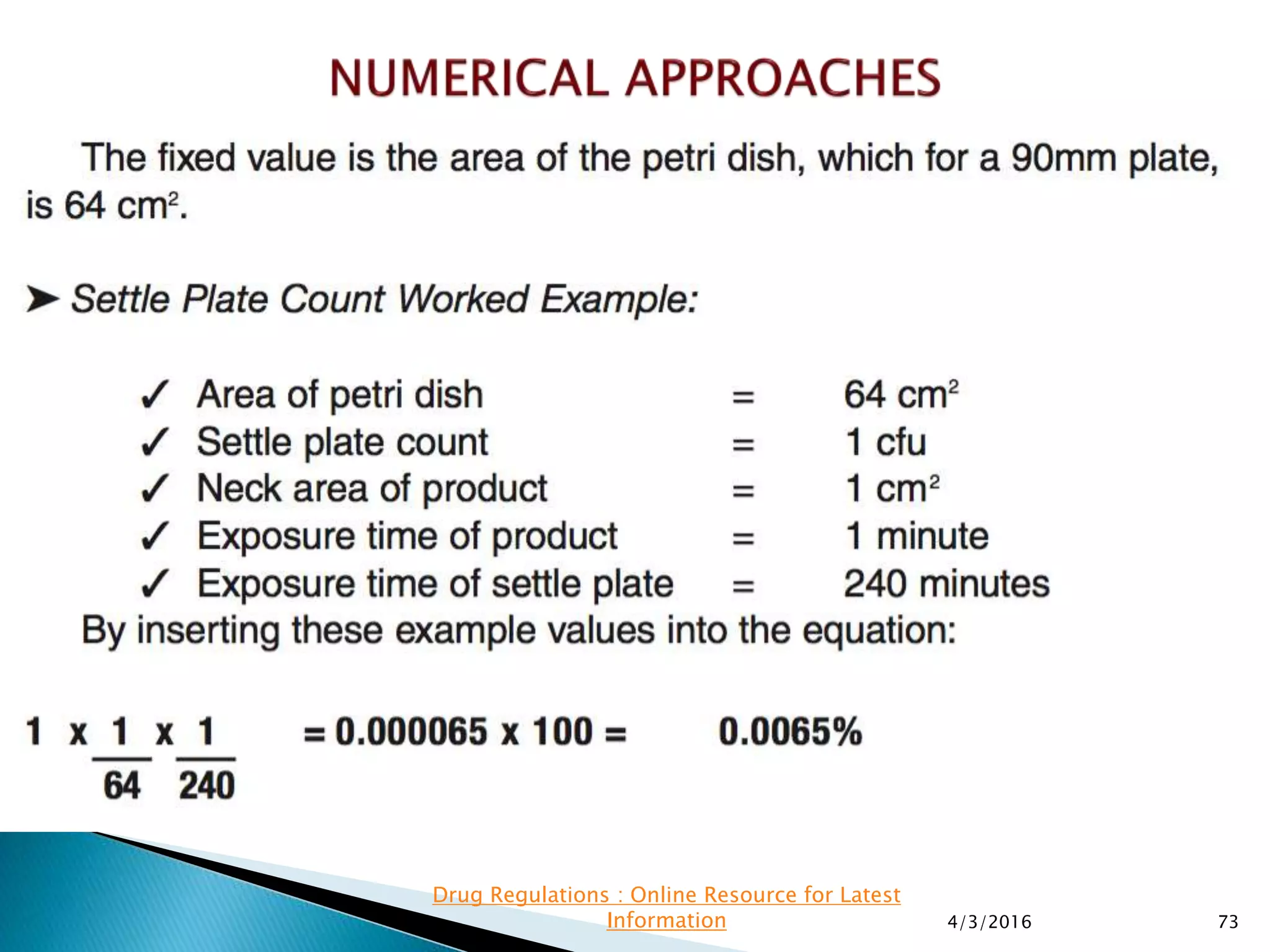
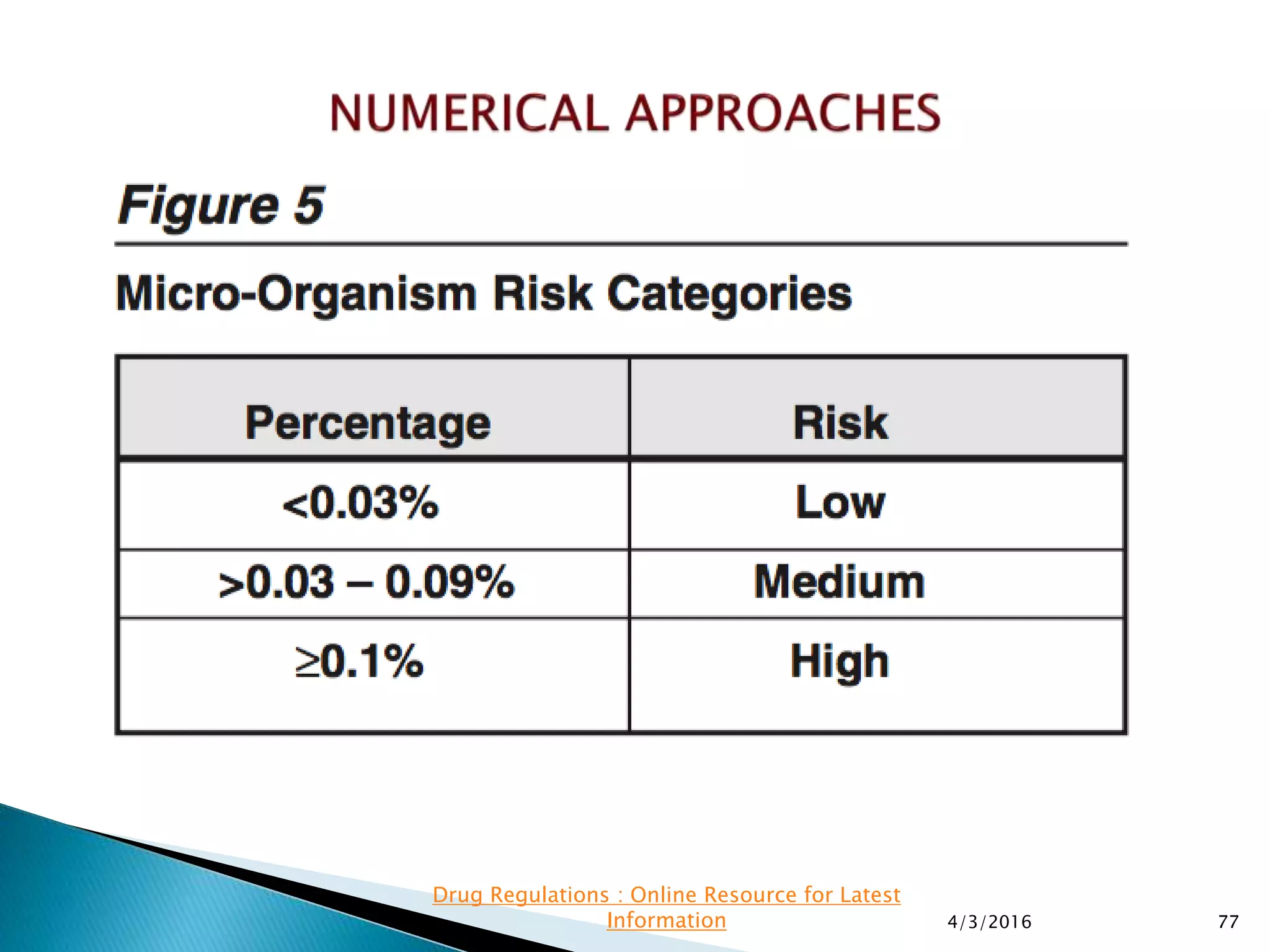
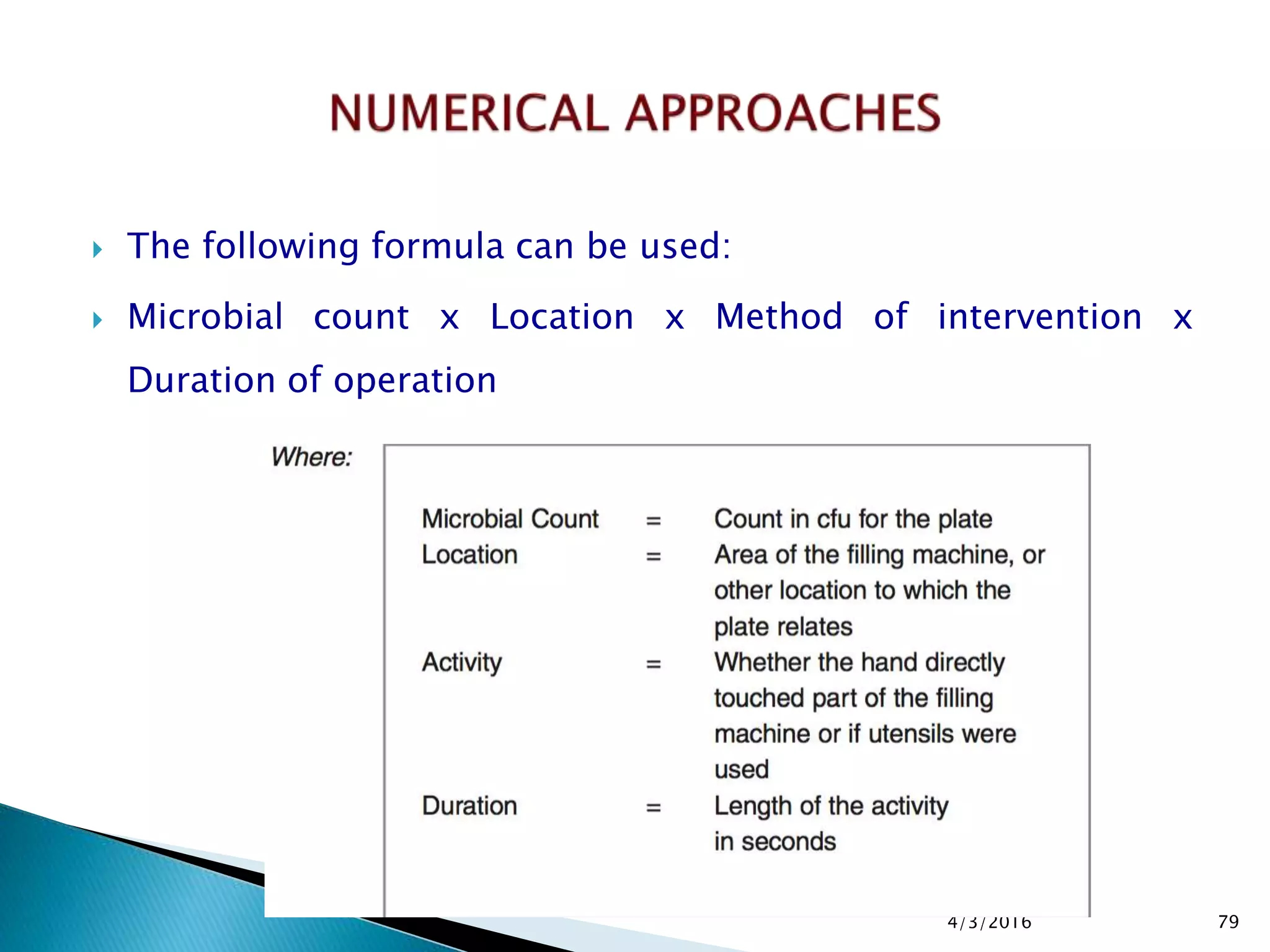
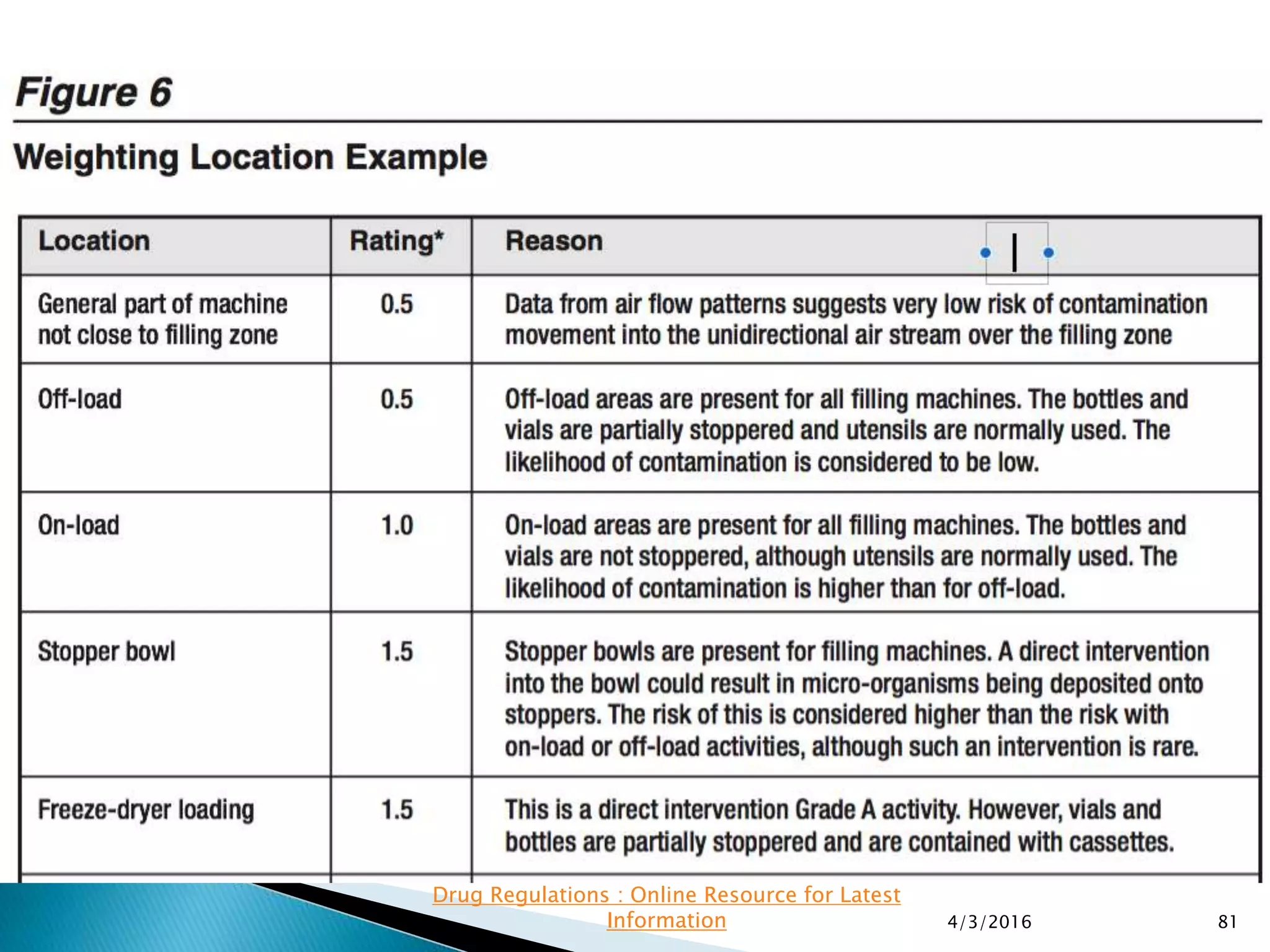
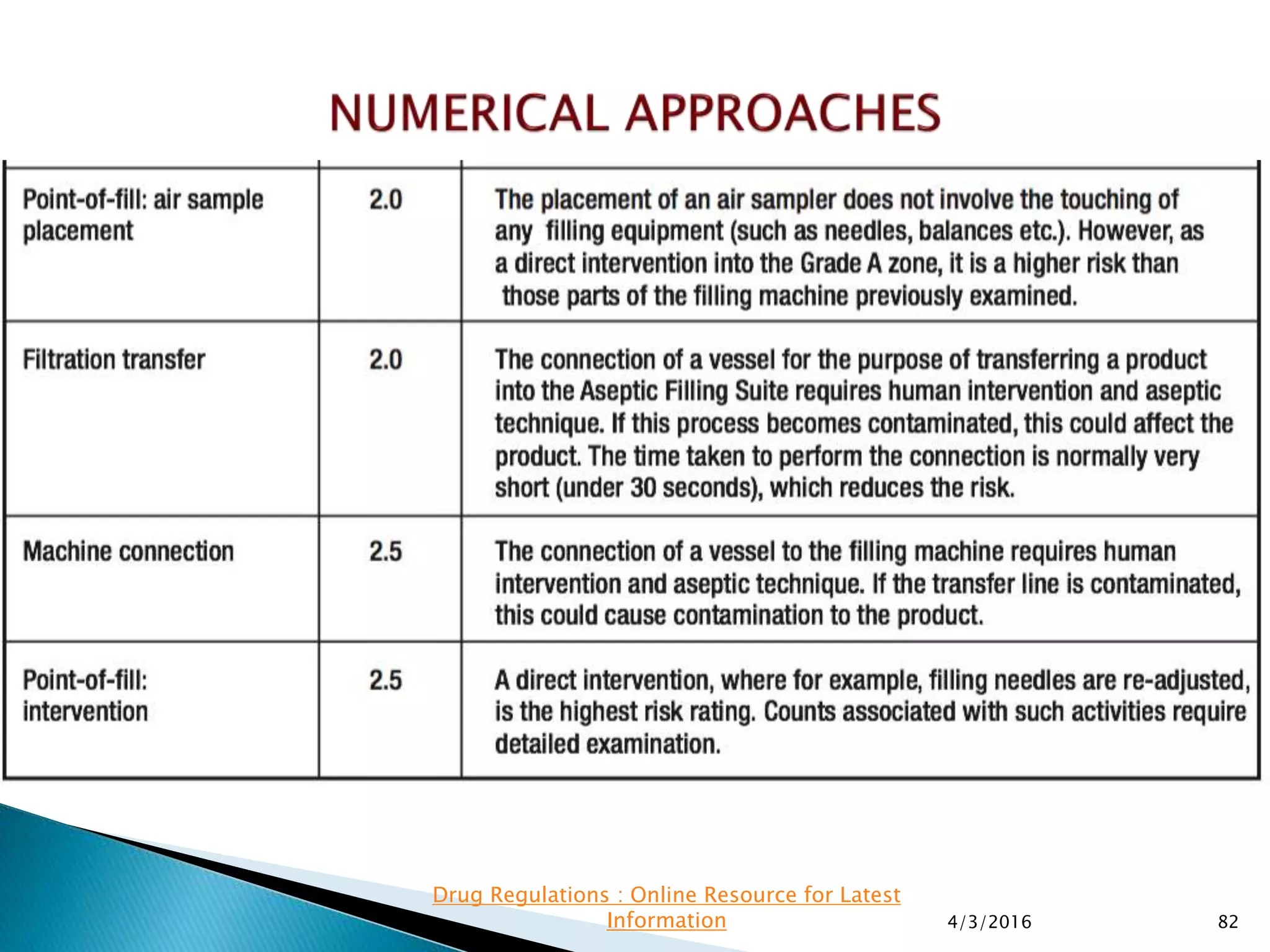
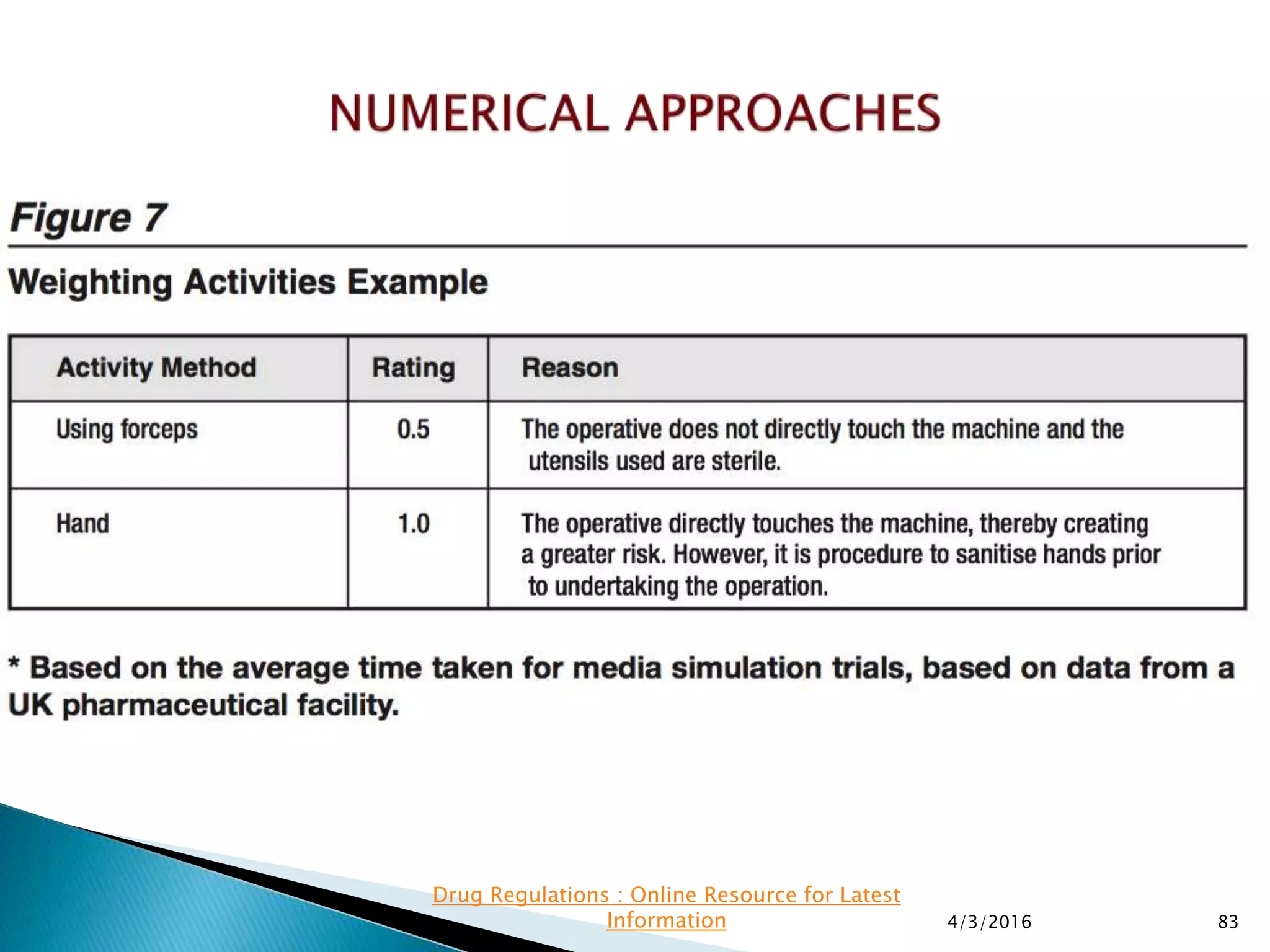
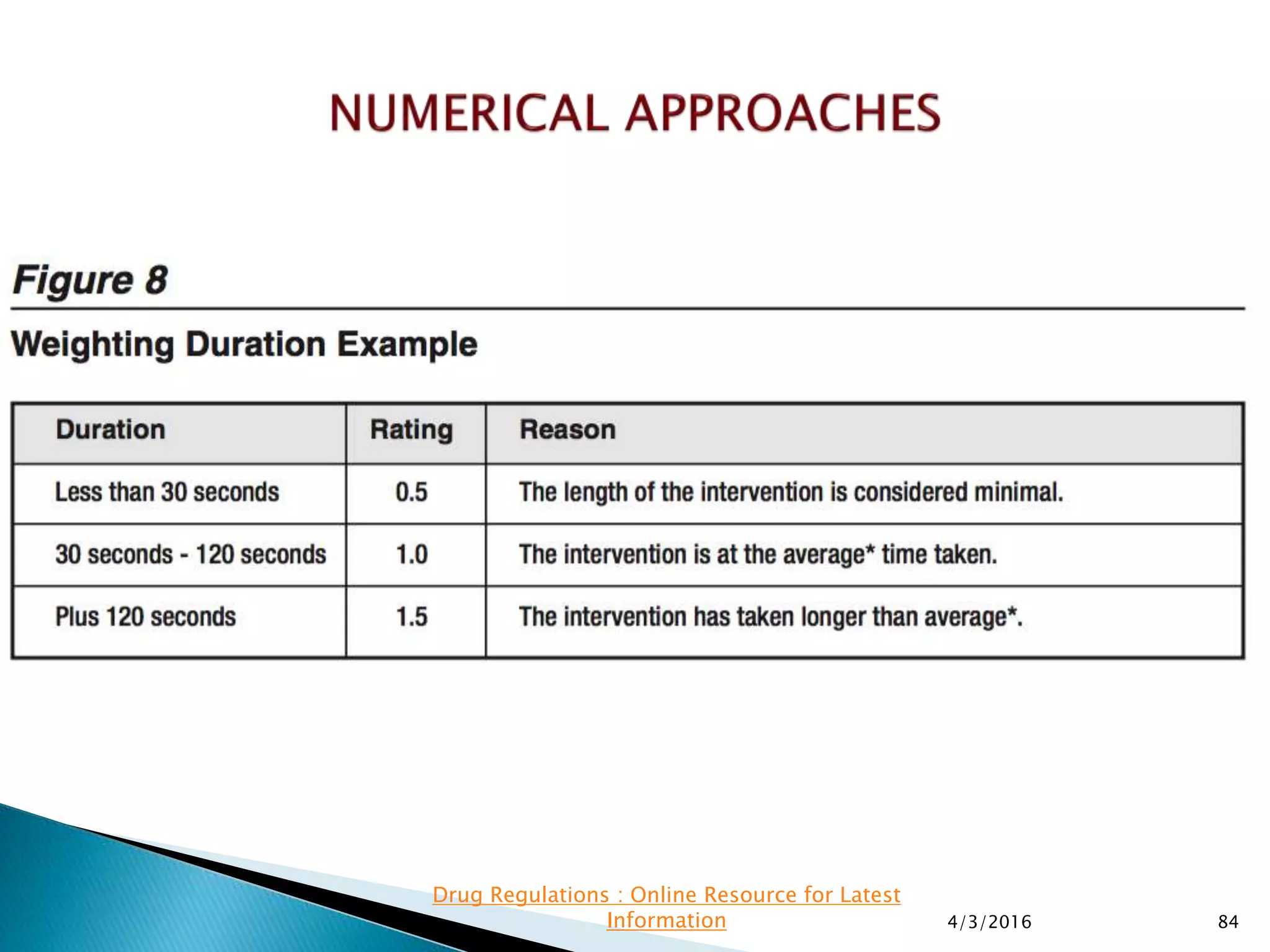
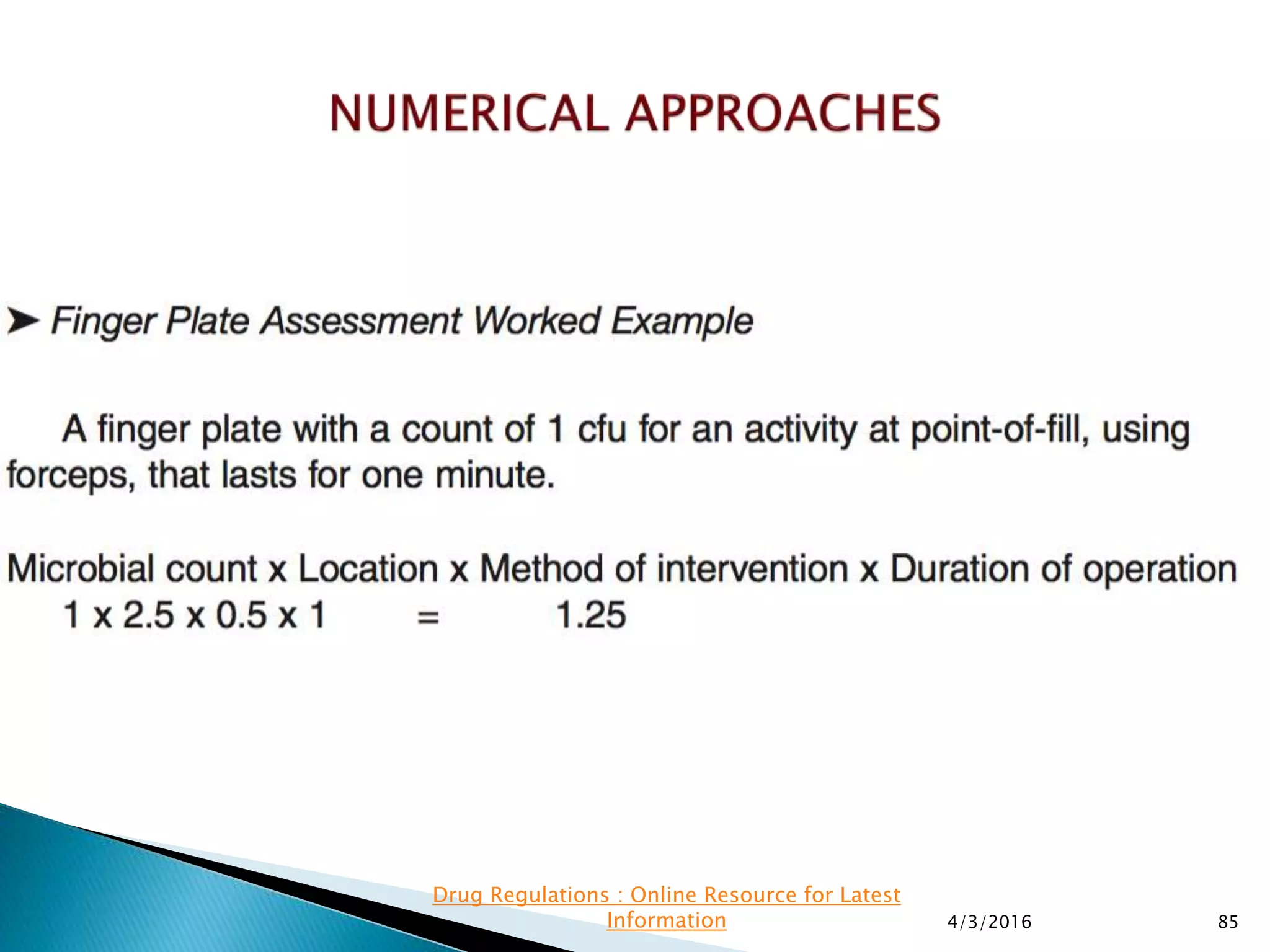
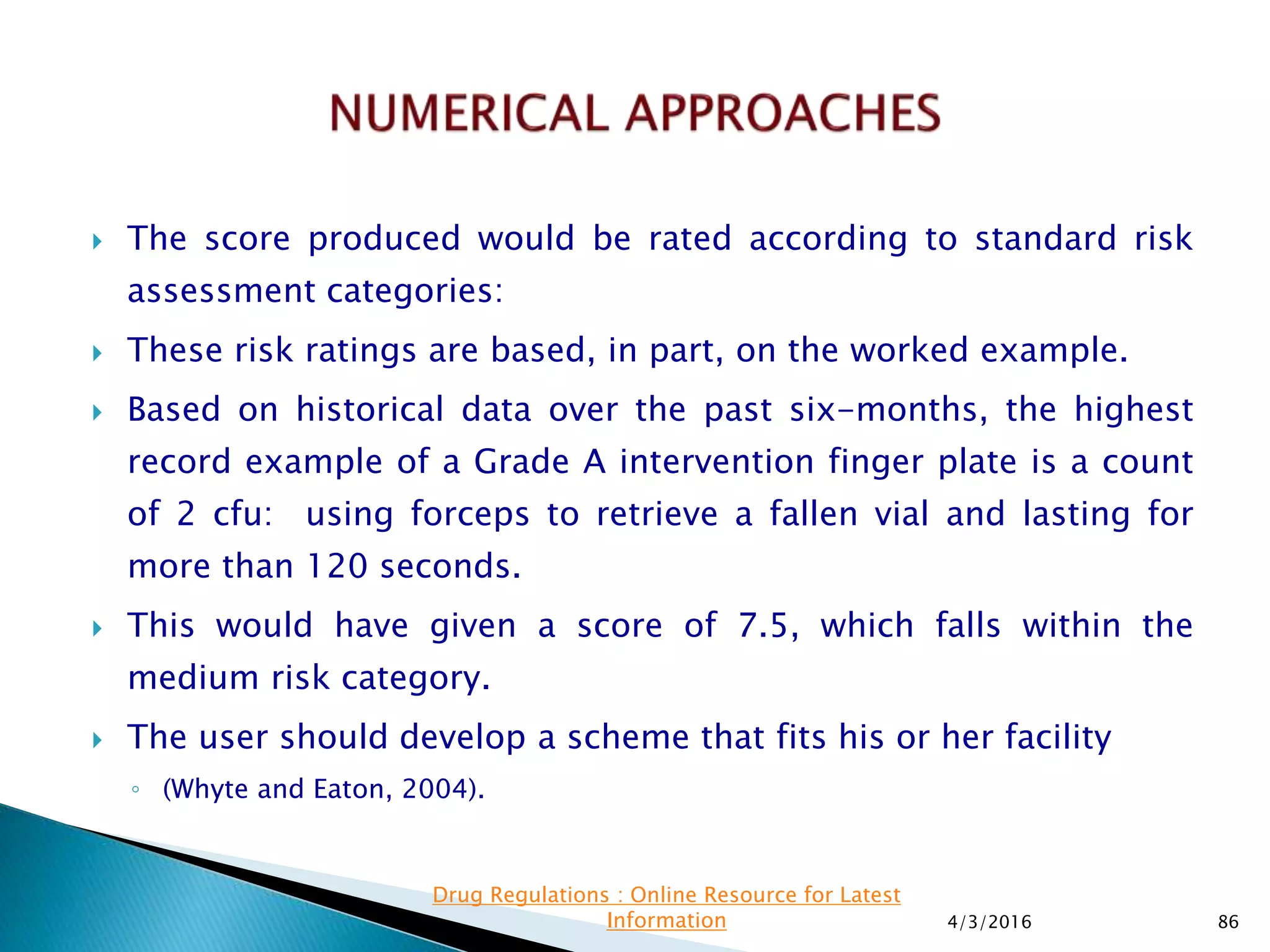
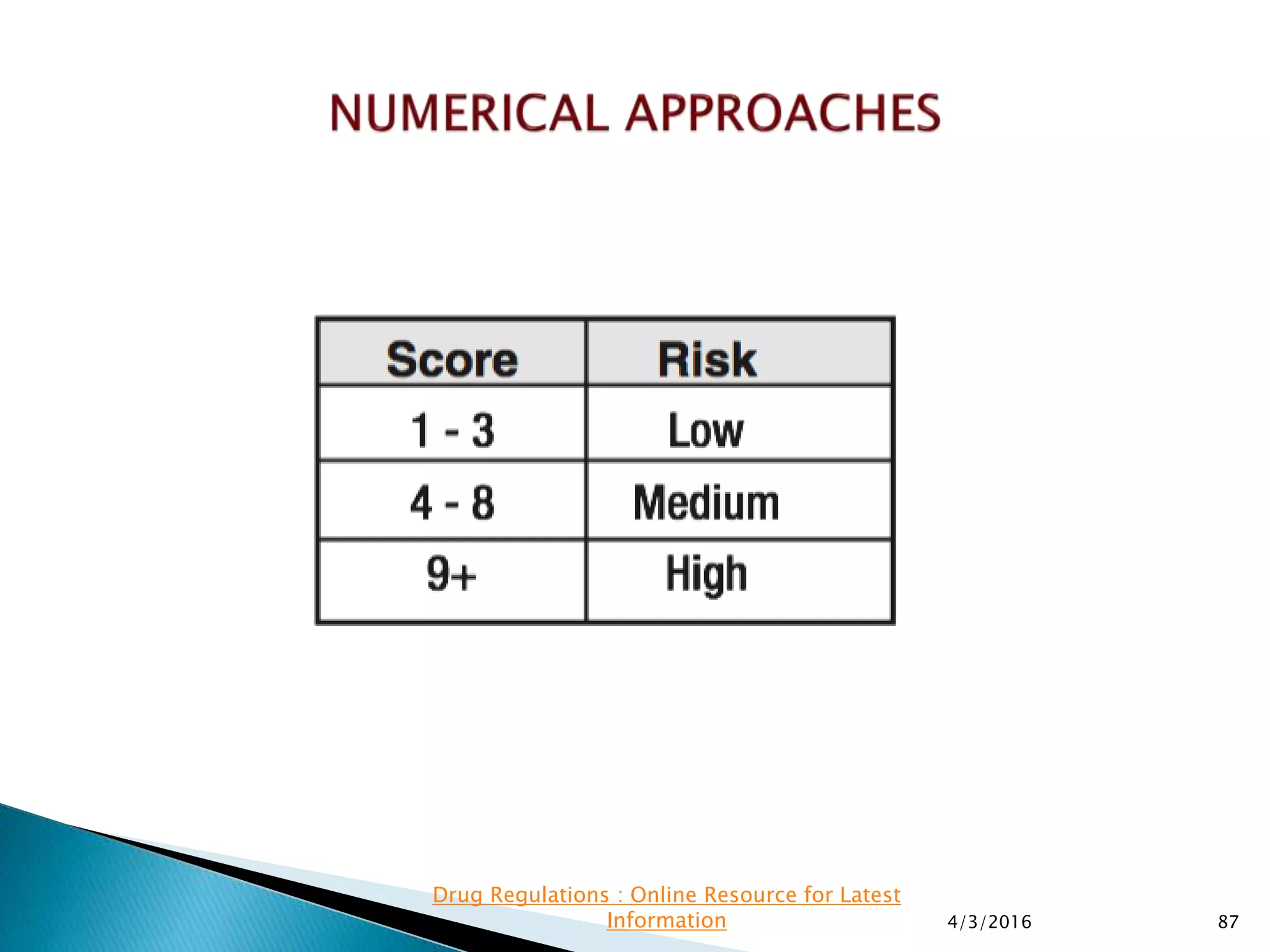
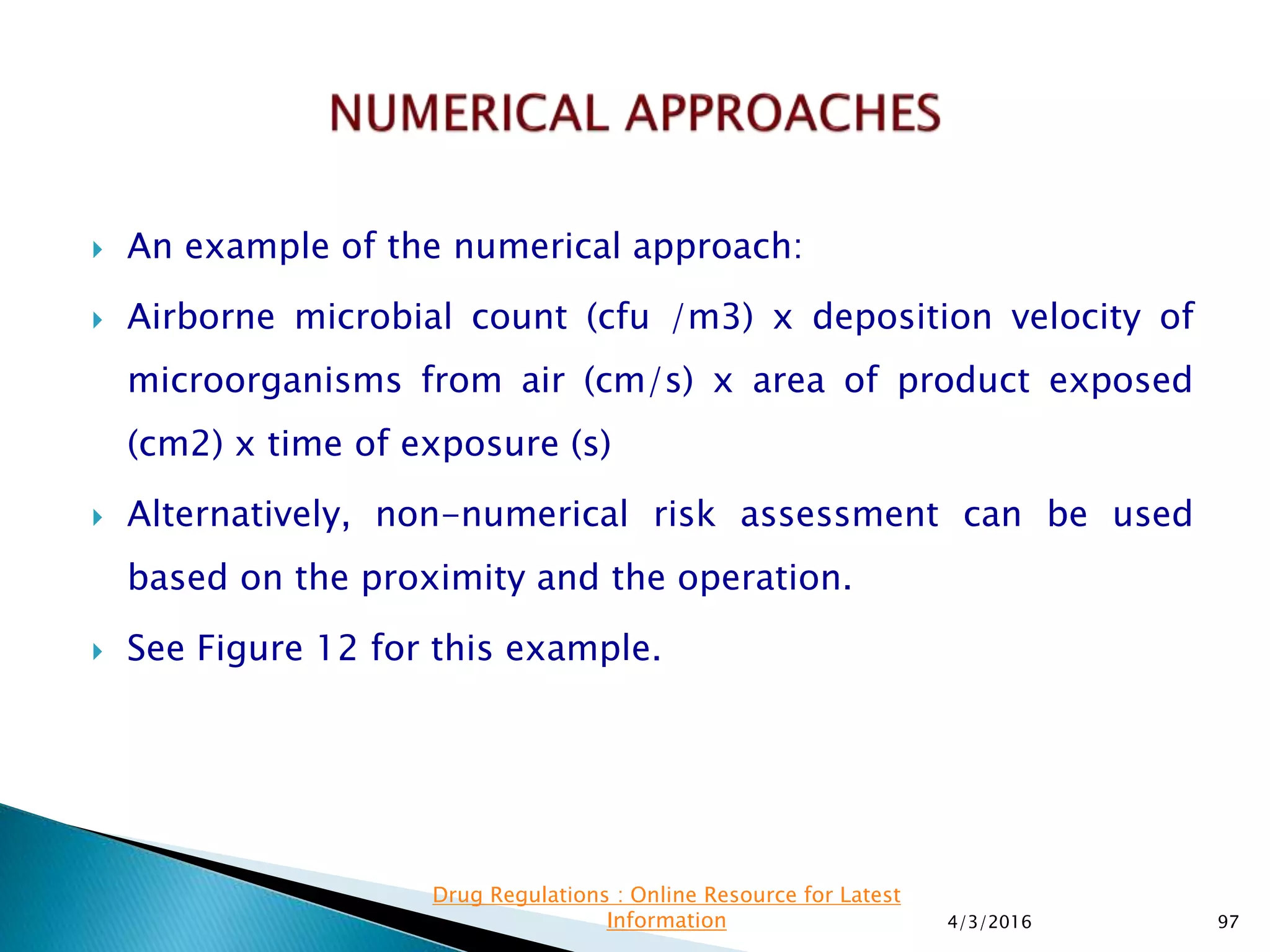
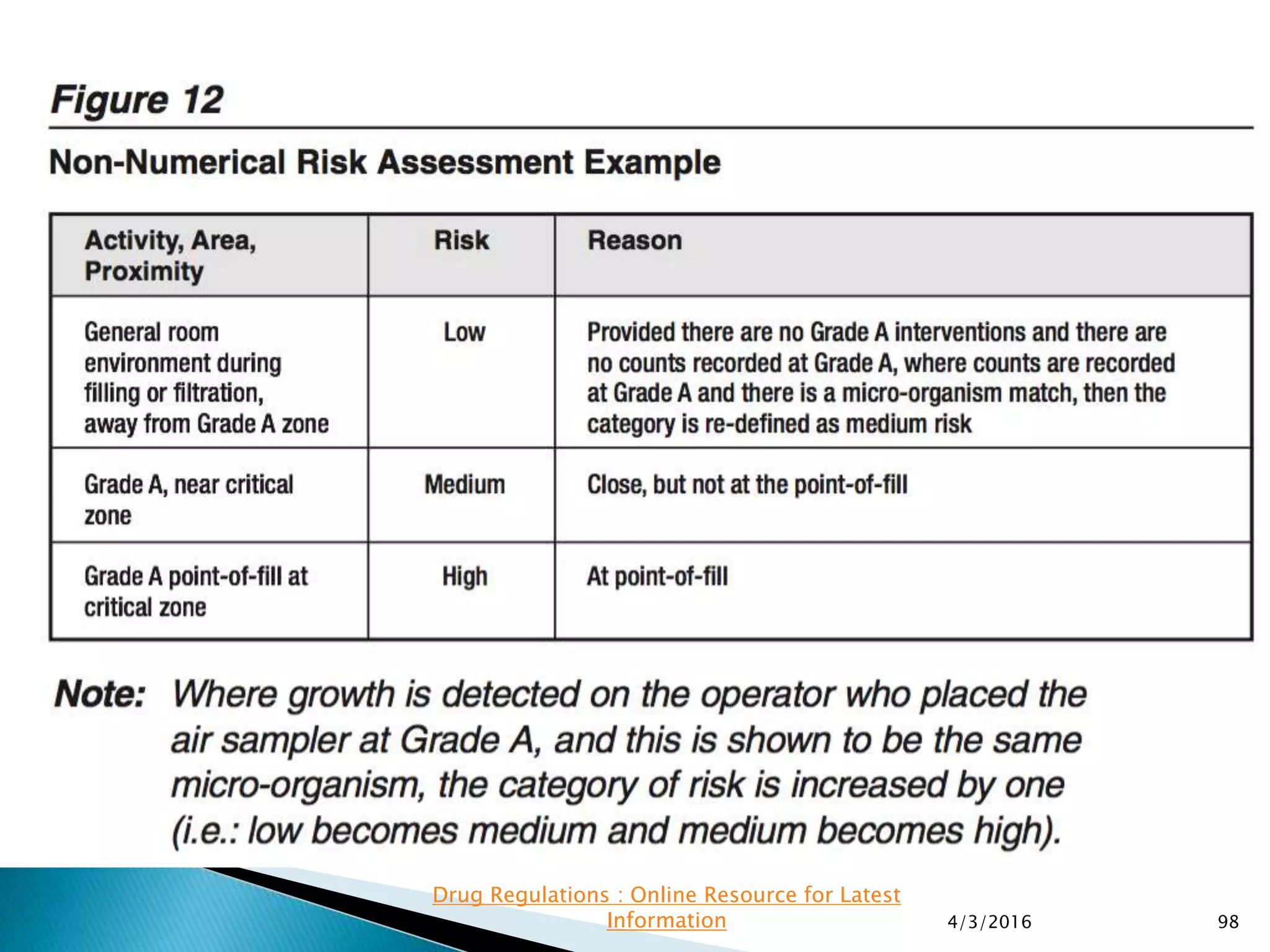
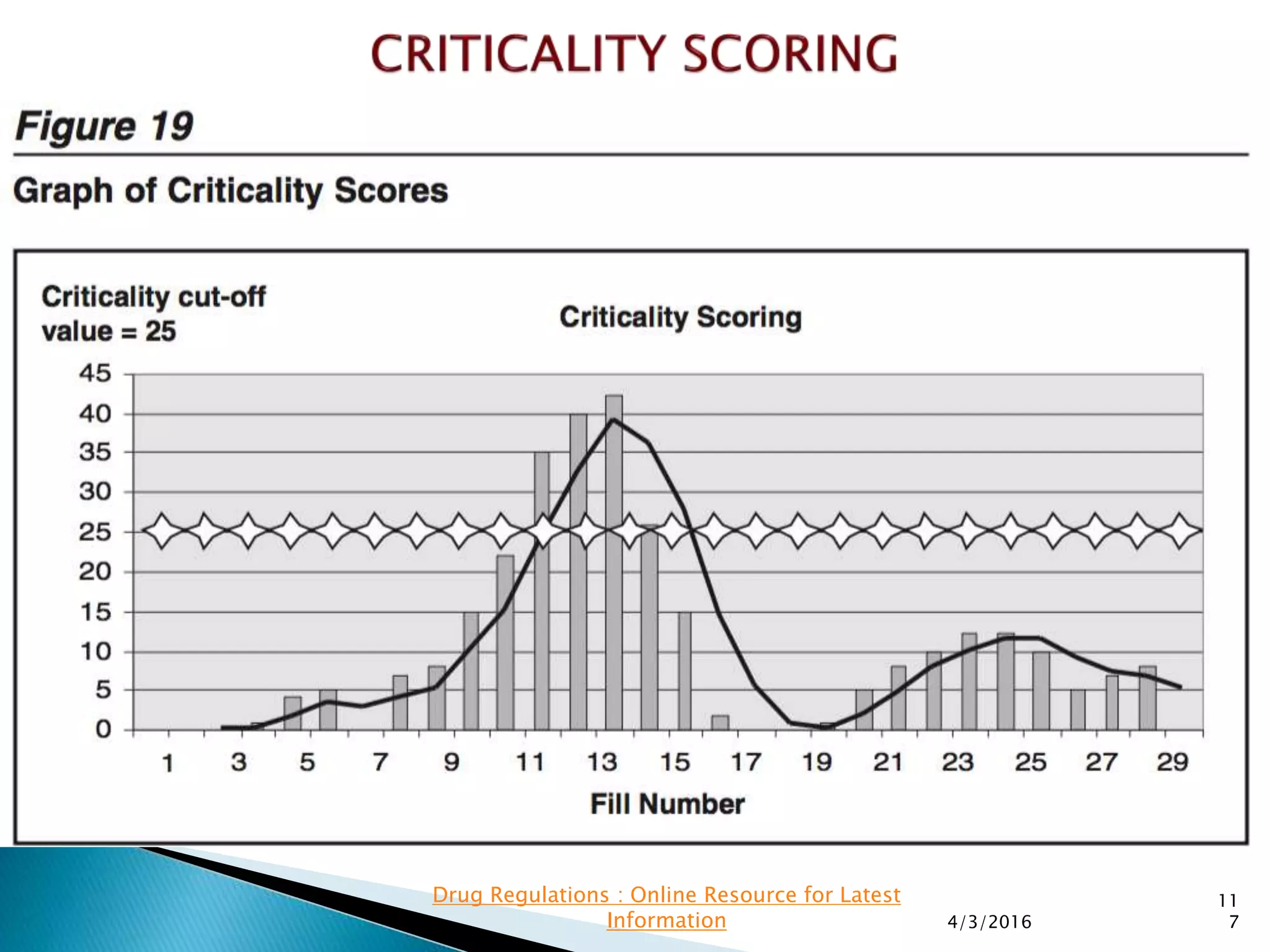
This presentation from the non-profit organization 'Drug Regulations' discusses environmental monitoring in cleanrooms, focusing on risk assessment for determining monitoring frequencies based on criticality factors. It emphasizes the use of various risk assessment tools like HACCP and FMEA to evaluate contamination risks and establish monitoring protocols. The document outlines how to adapt monitoring strategies according to environmental control needs and the importance of continuous review and adjustment based on obtained data.