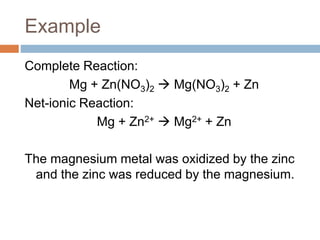
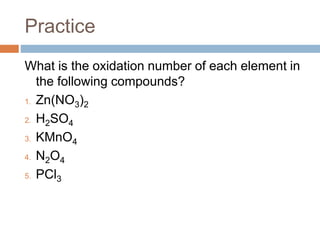
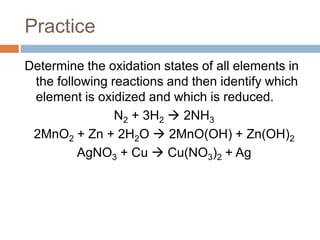
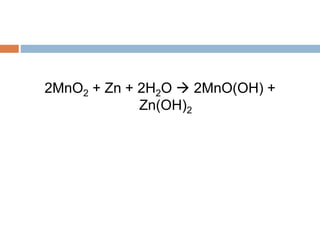
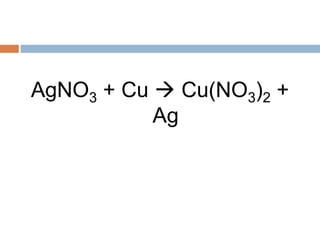
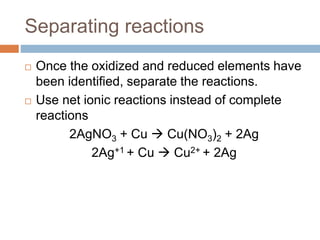
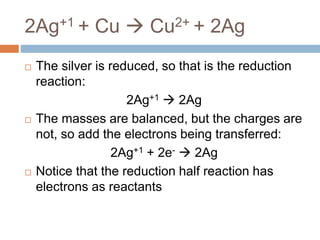
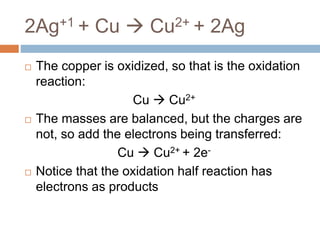
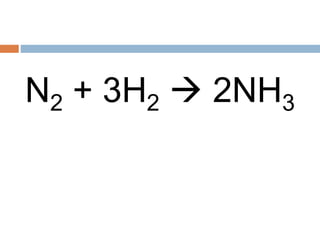The document discusses oxidation-reduction (redox) reactions, where there is a transfer of electrons between reactants. It defines oxidation as the loss of electrons and reduction as the gain of electrons. An example redox reaction and its net ionic form are provided. The document explains how to determine the oxidation states of elements and identifies common oxidation states of nonmetals. It describes how to write half-reactions by separating a redox reaction into its oxidation and reduction components.