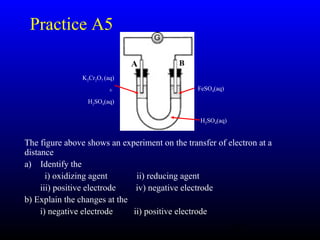
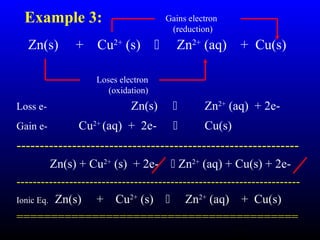
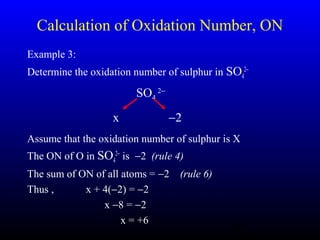
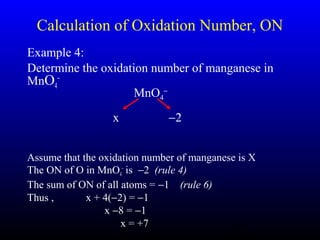
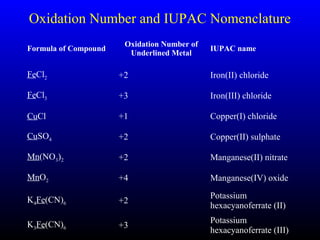
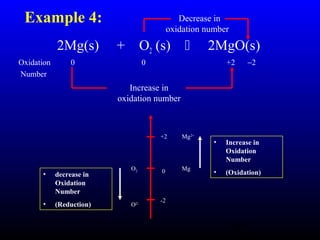
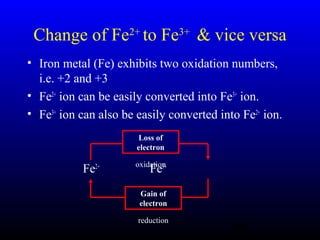
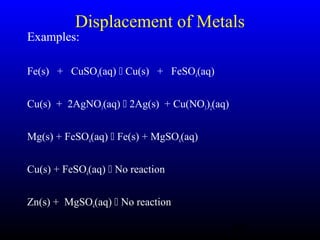
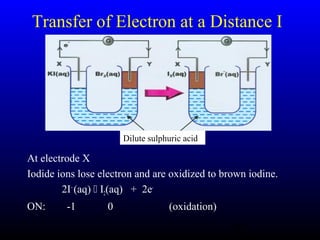
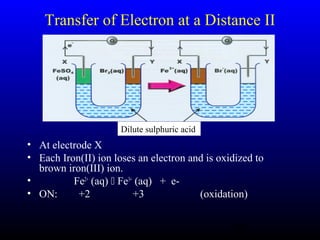
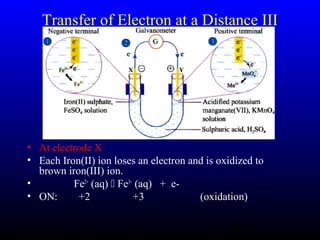
The document discusses oxidation, reduction, and redox reactions. It defines oxidation as gain of oxygen, loss of hydrogen, or loss of electrons, and reduction as the opposite. Redox reactions involve both oxidation and reduction occurring simultaneously through the transfer of electrons between reactants.













































![59
Overall ionic equation:
anode 5[Fe2+
(aq) Fe3+
(aq) + e-]
cathode MnO4
−
(aq) + 8H+
(aq) + 5e- Mn2+
(aq) + 4H2O(l)
………………………………………………………………………
5Fe2+
(aq) + MnO4
−
(aq) + 8H+
(aq) 5Fe3+
(aq)+ Mn2+
(aq) + 4H2O(l)
Substance oxidized : iron(II) ion, Fe2+
Substance reduced : manganese(VII) ion, MnO4
−
Oxidizing agent : manganese(VII) ion, MnO4
−
Reducing agent : iron(II) ion, Fe2+](https://image.slidesharecdn.com/3aredoxreaction-150409190015-conversion-gate01/85/3-a-redox-reaction-59-320.jpg)