Embed presentation
Download to read offline








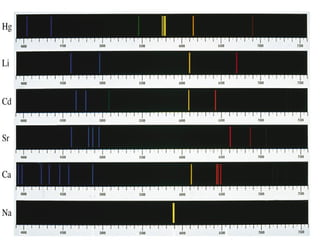

The document discusses the Bohr model of the hydrogen atom. It explains that: 1) Electrons orbit the nucleus in fixed energy levels called orbits. 2) Electrons can move between energy levels by gaining or losing discrete amounts of energy. 3) Electrons cannot exist between energy levels, which are like rungs on a ladder with the lowest level closest to the nucleus.










