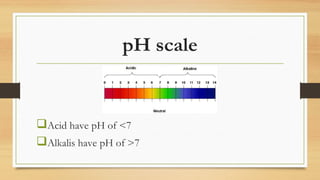
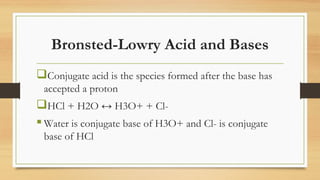
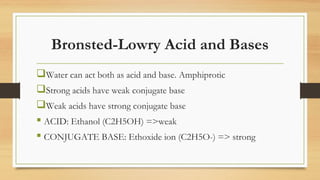
This document discusses acids and bases. It defines acids as substances that produce H+ ions in water and have a pH less than 7. Acids have properties such as turning litmus red and reacting with metals. Bases are defined as substances that produce OH- ions in water and have a pH greater than 7. They have properties such as turning litmus blue and reacting with acids to form salt and water. Strong acids and bases are fully dissociated in water while weak acids and bases are only partially dissociated. The document also discusses Bronsted-Lowry and Lewis acid-base theories.