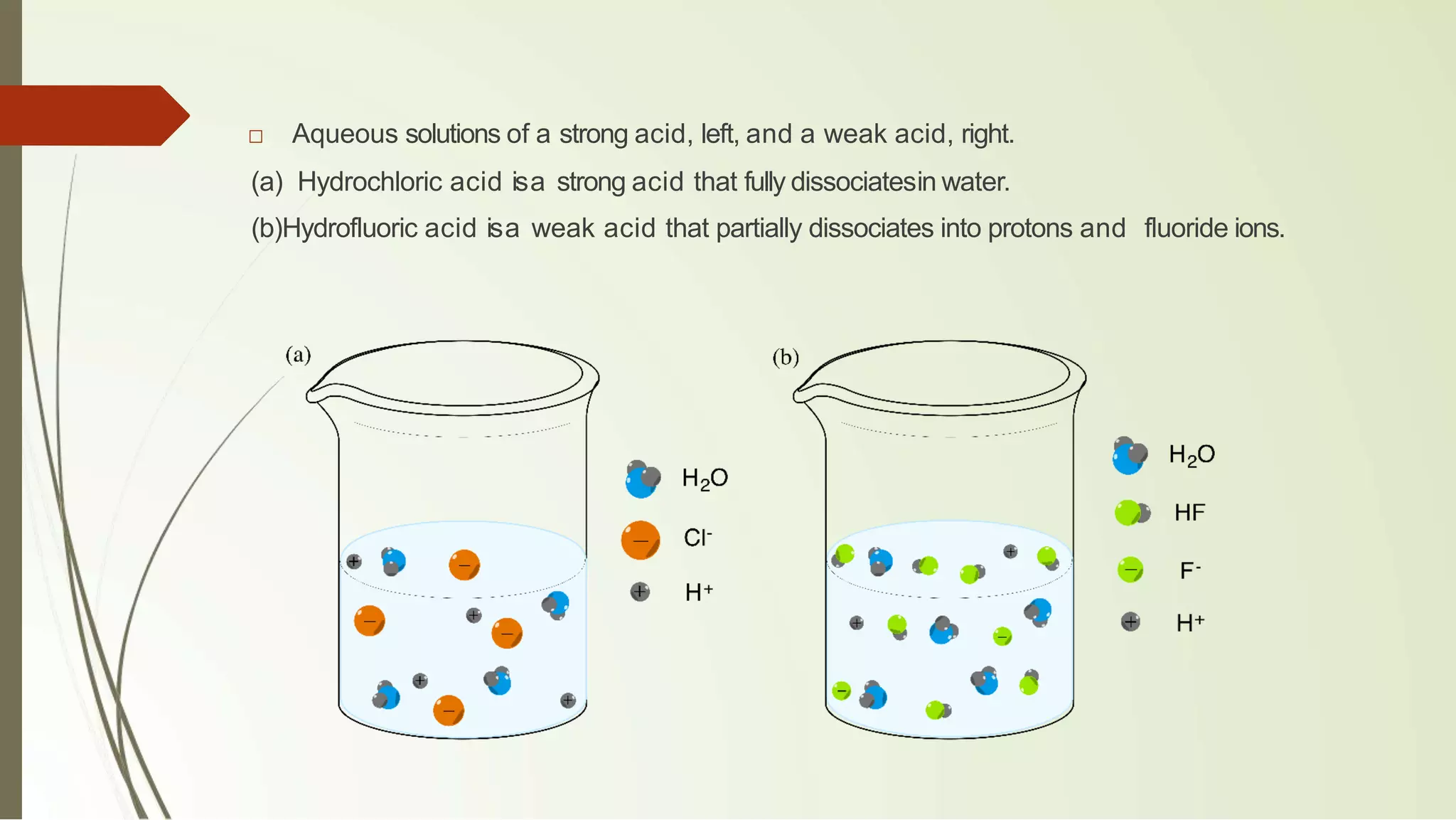
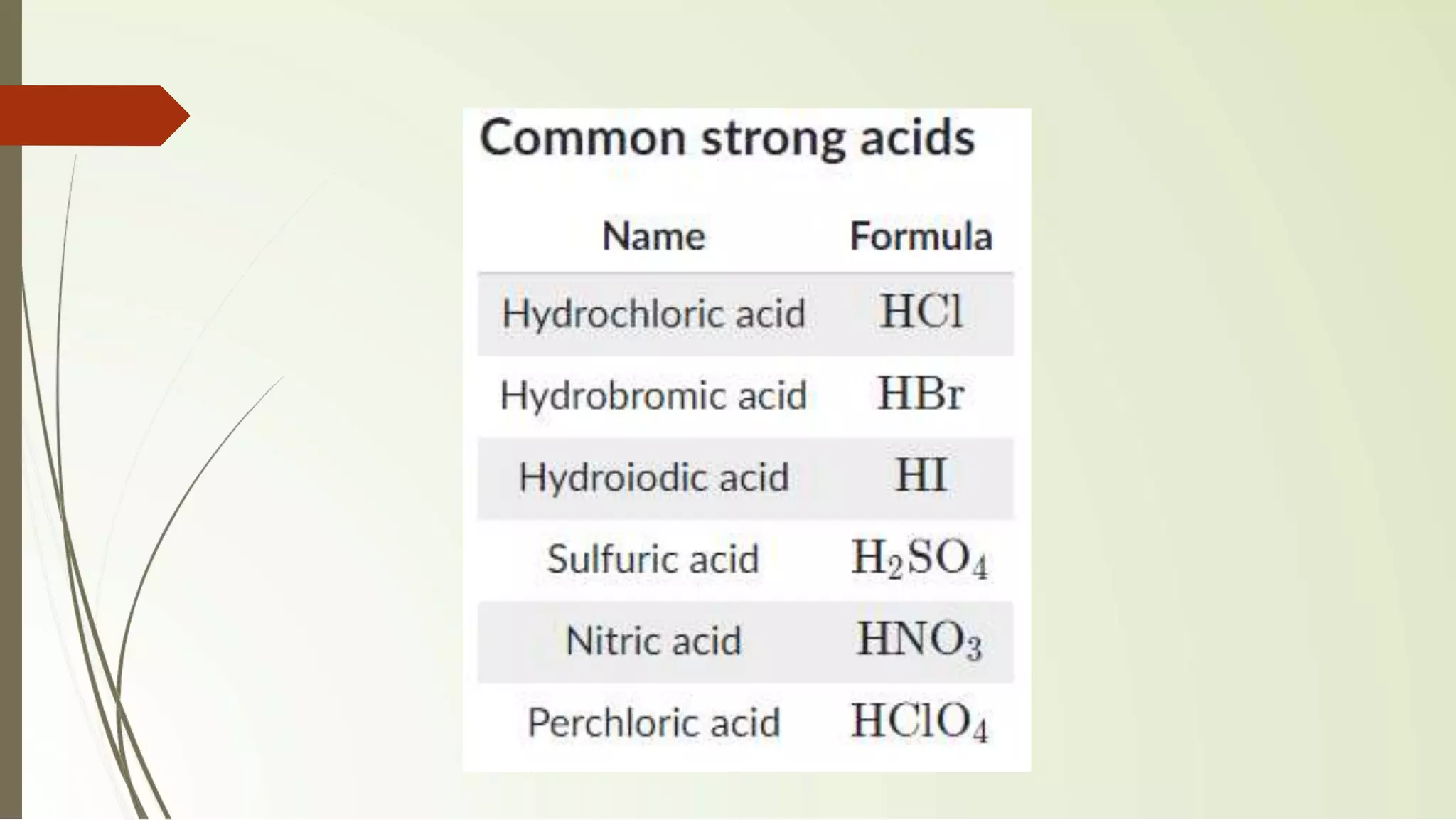
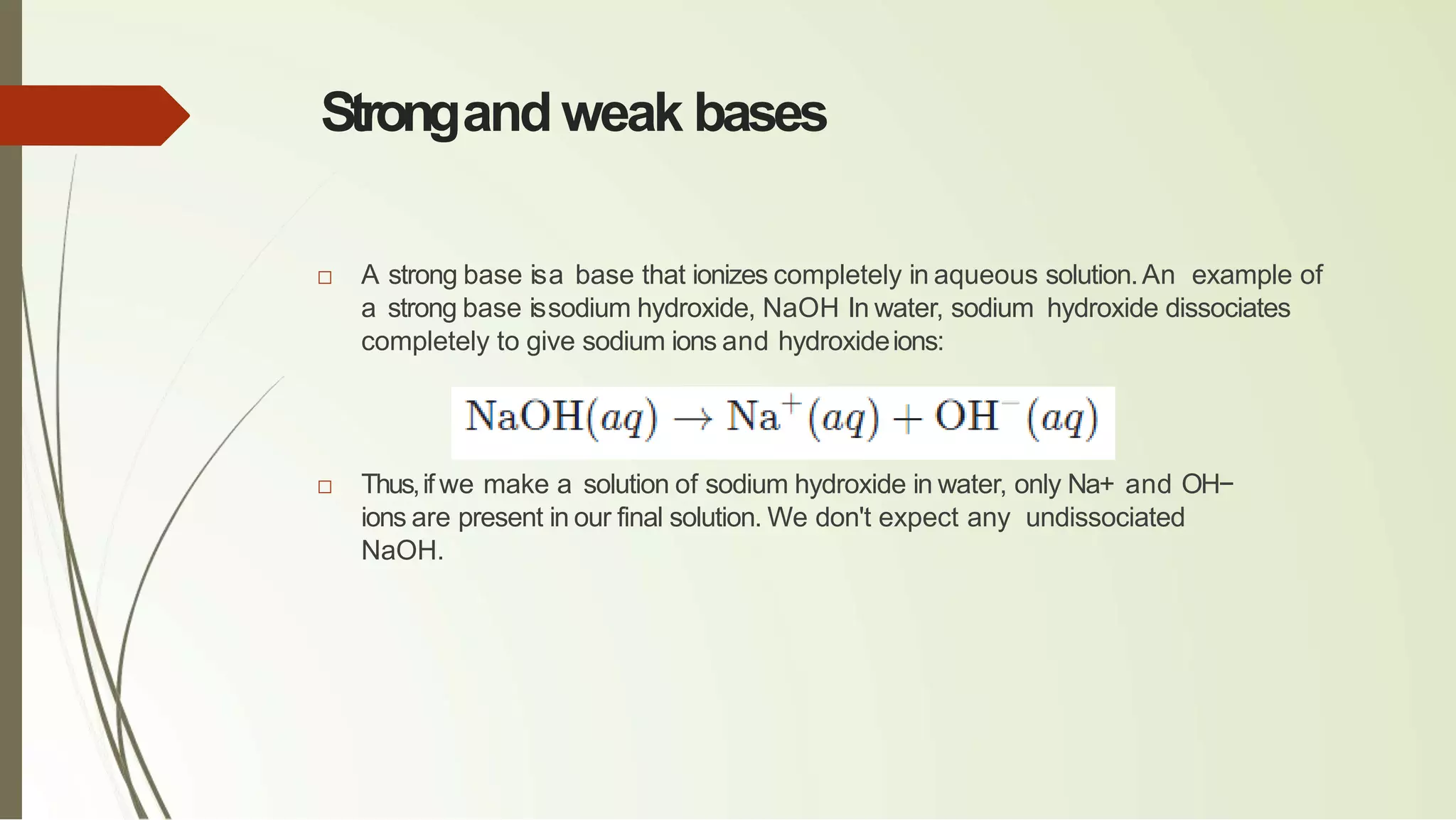
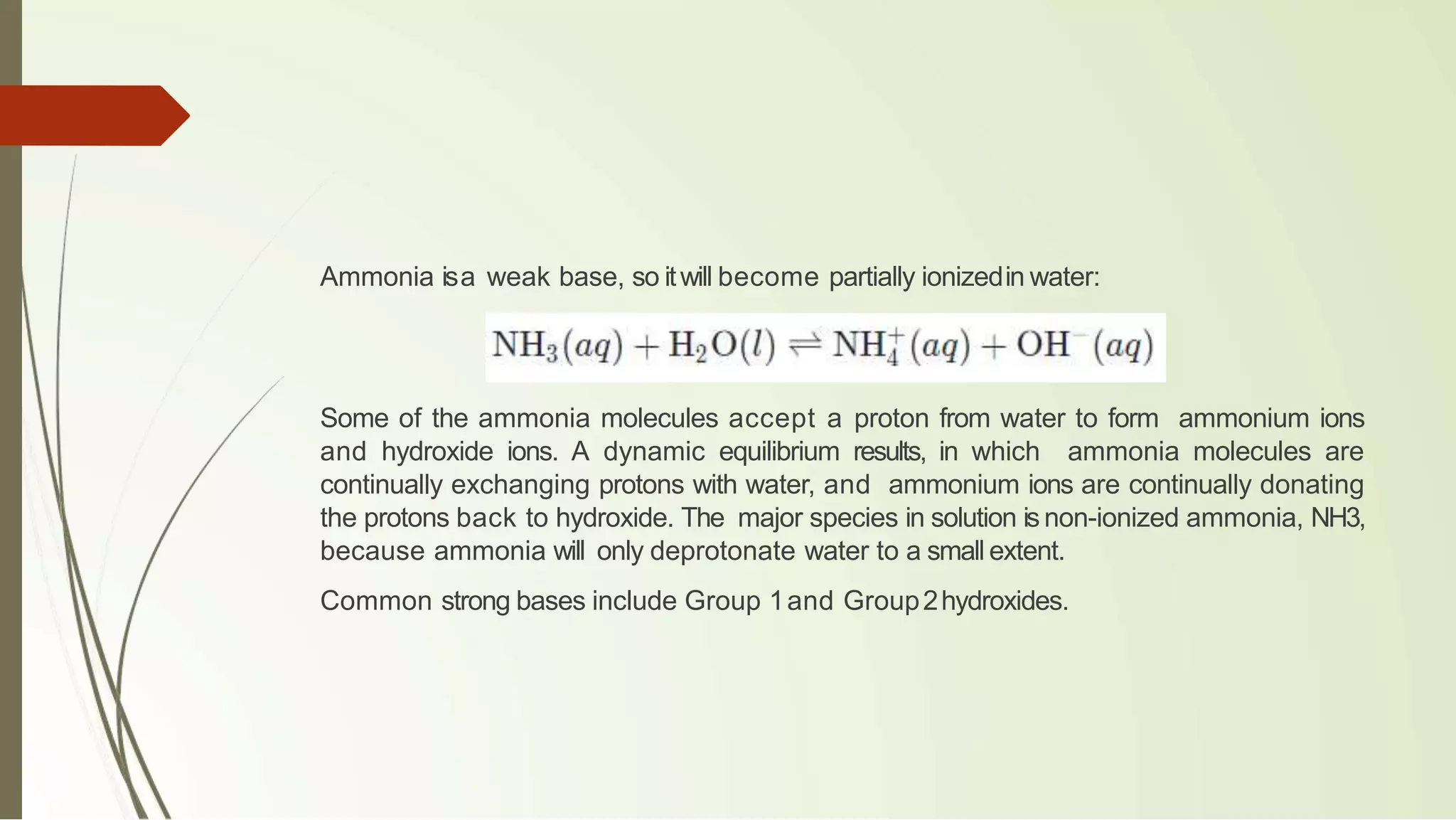
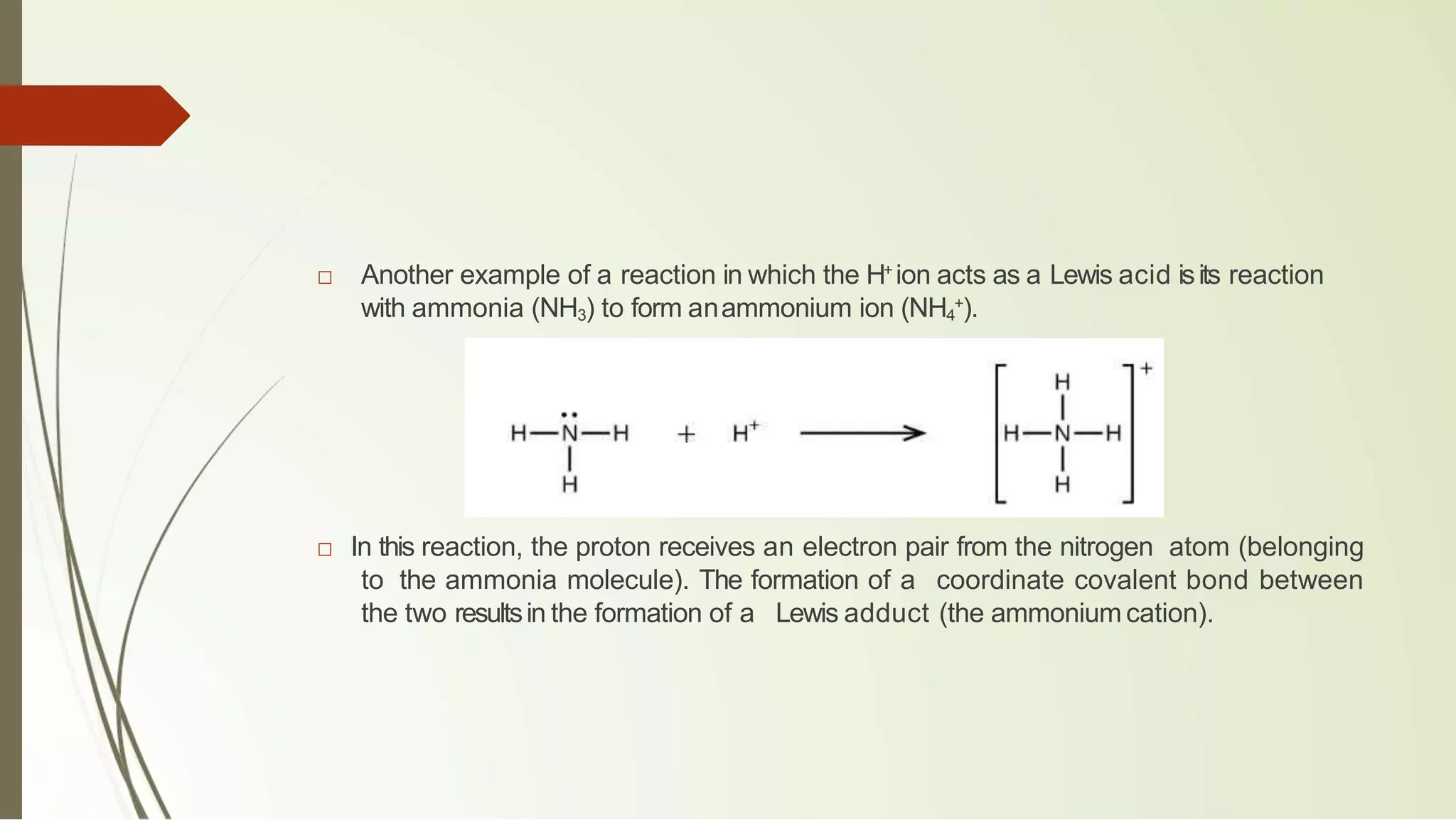
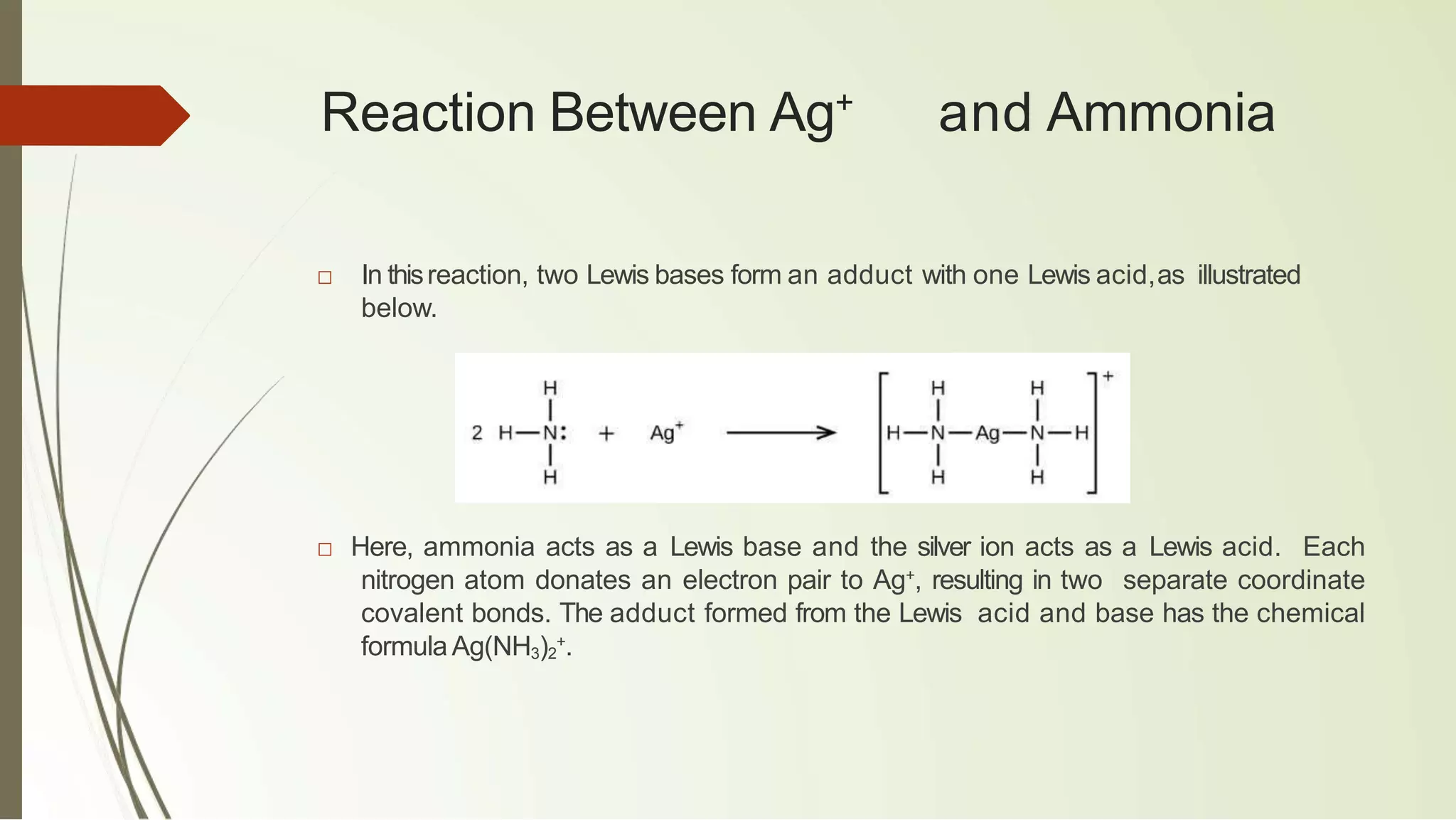
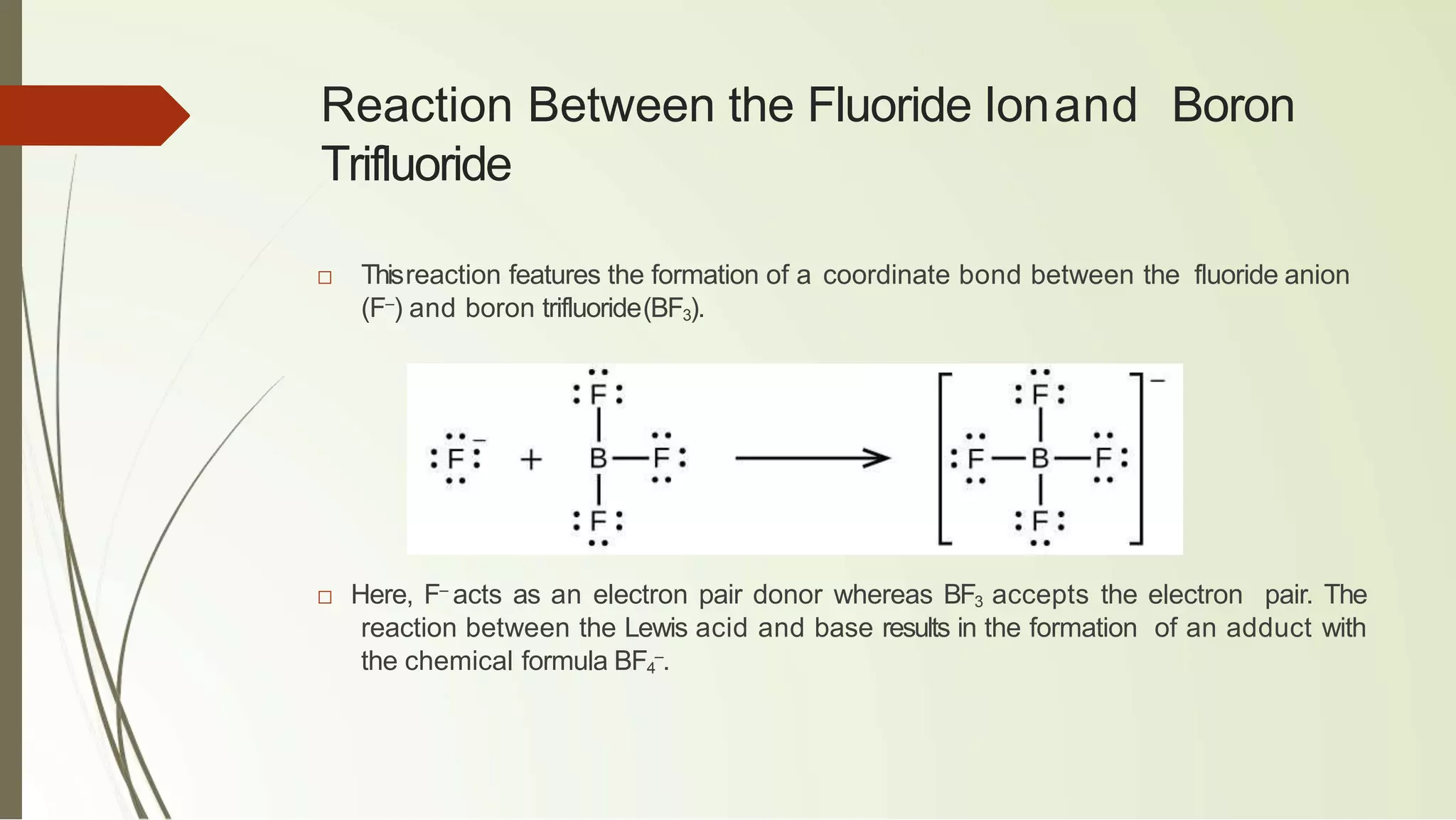
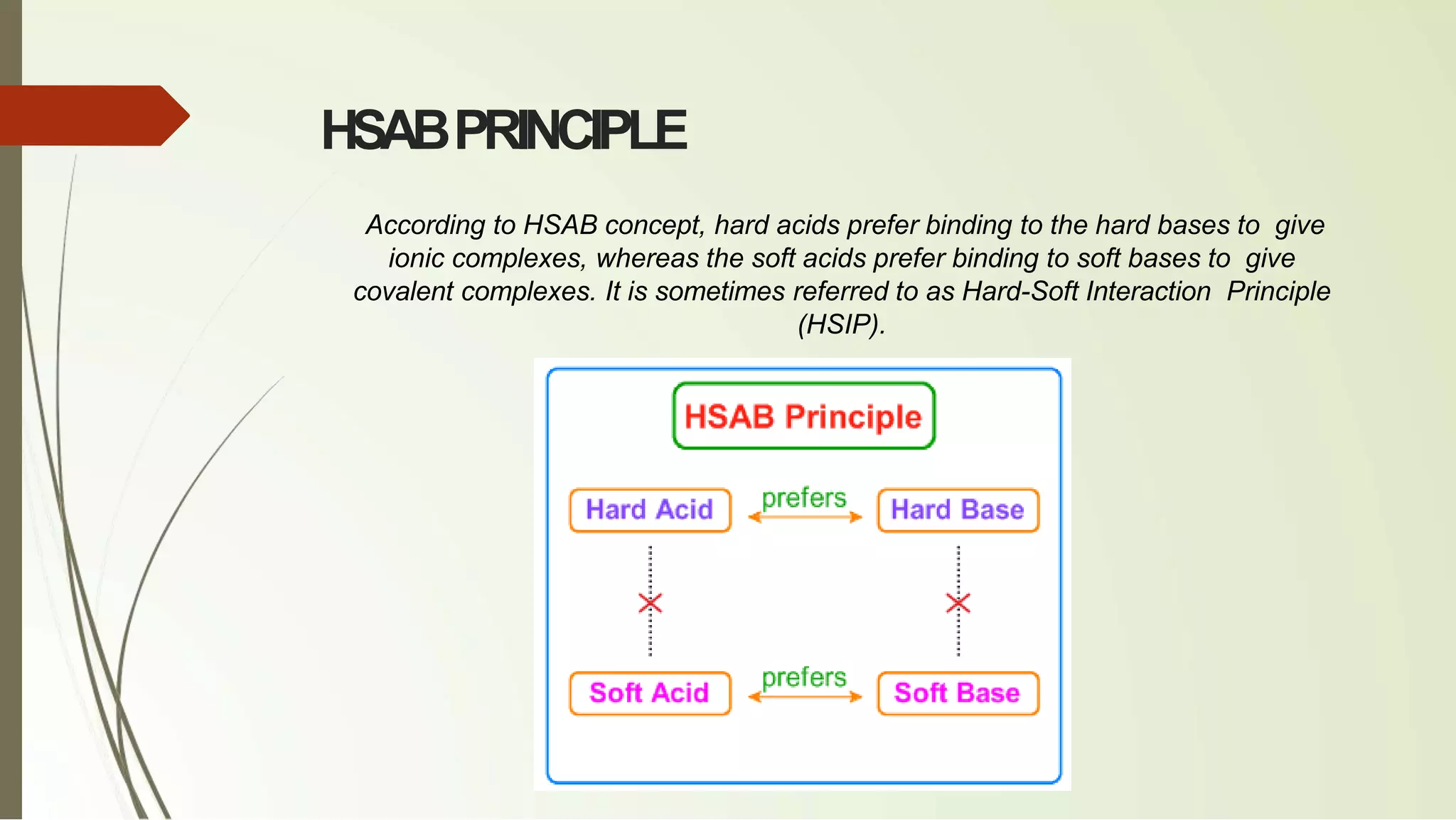
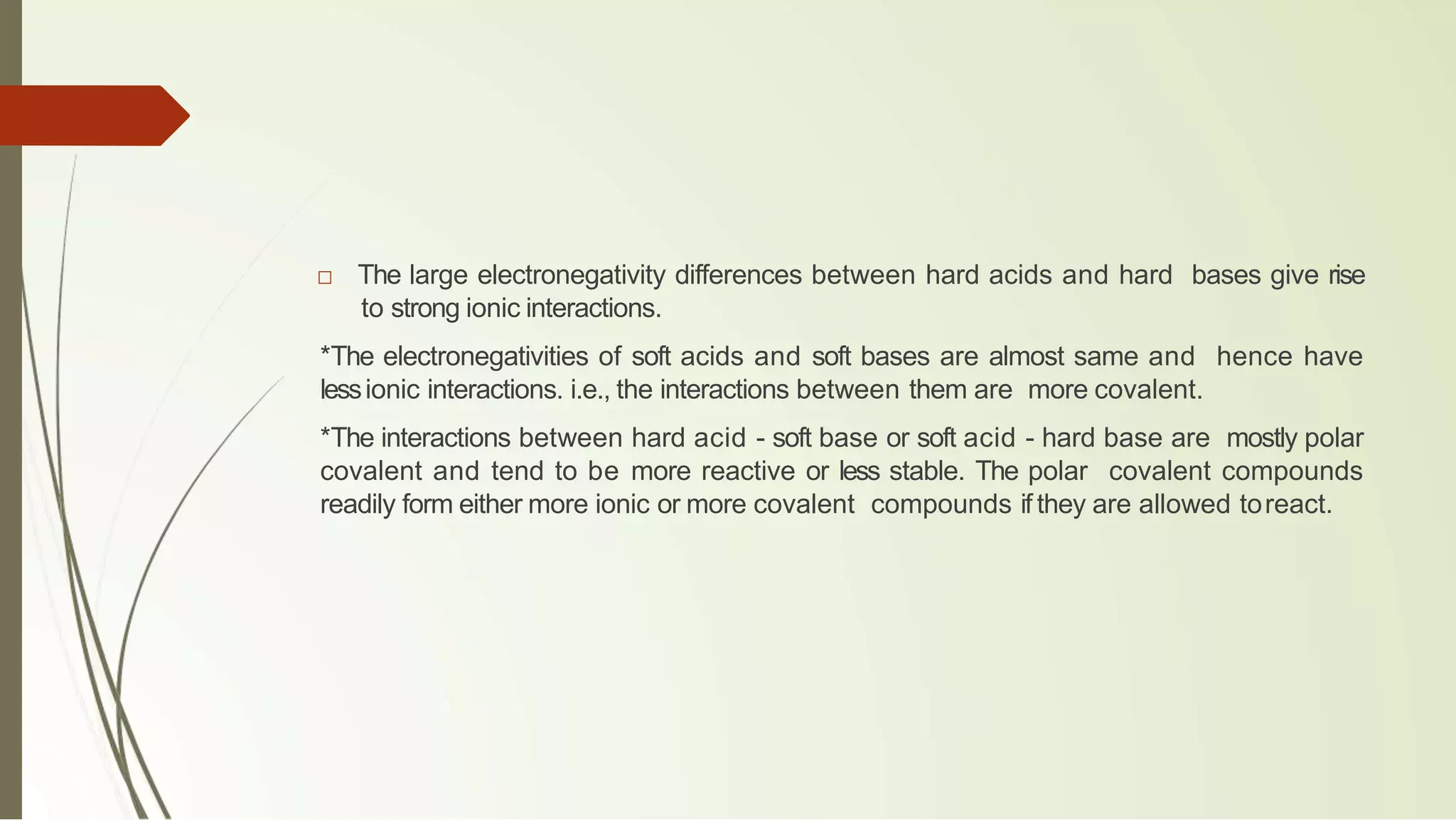
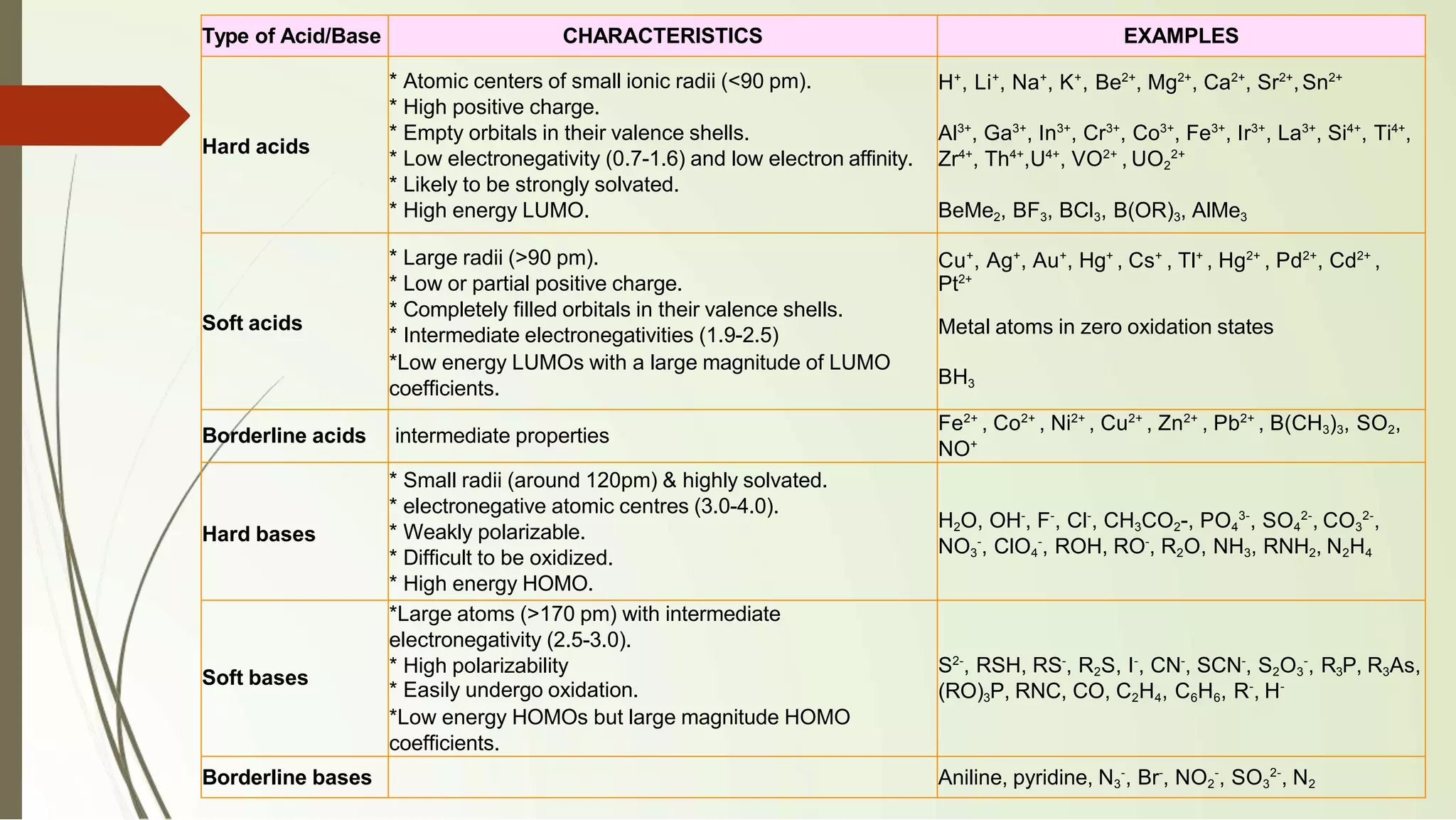
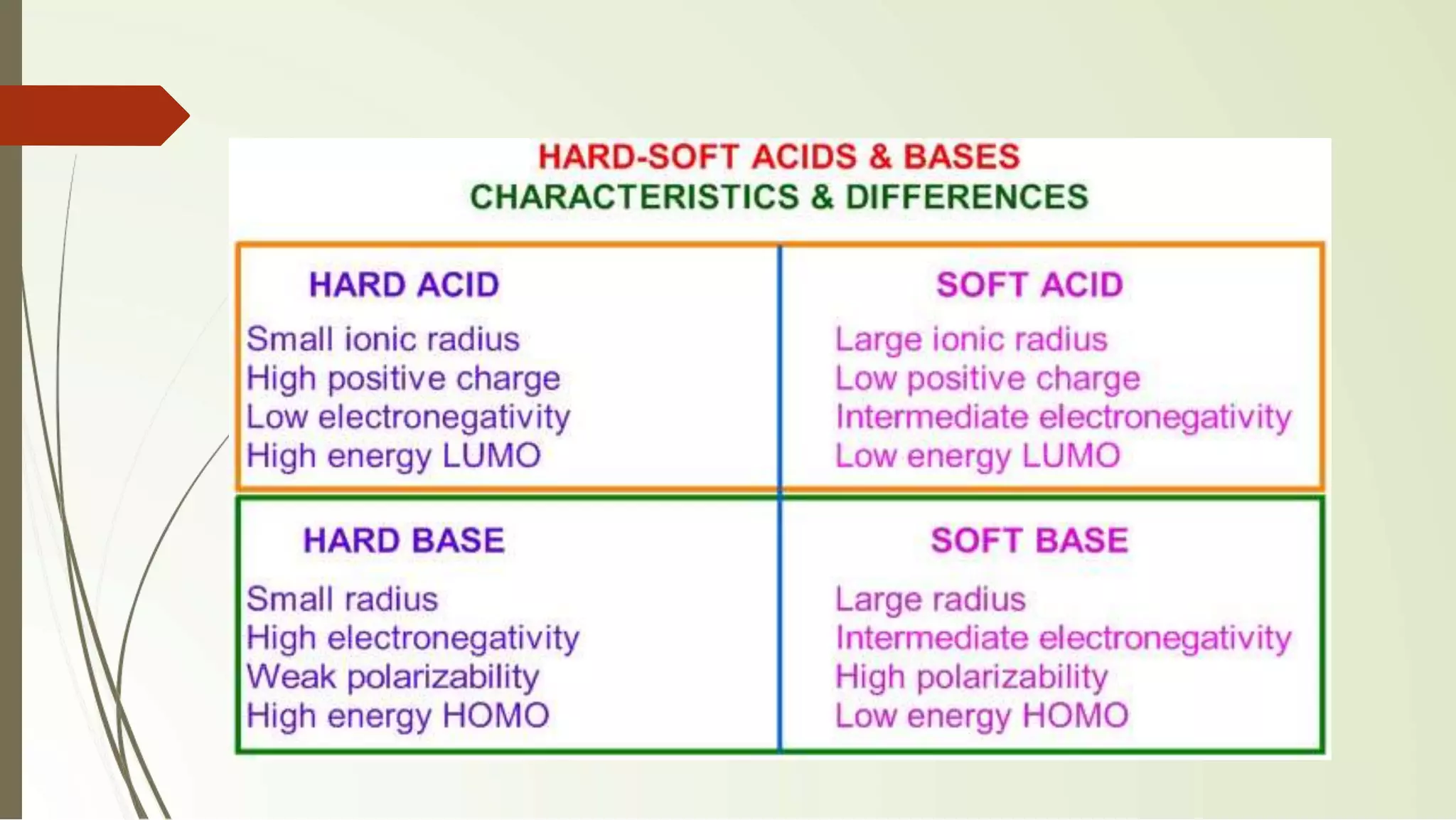
The document discusses the concepts of acids and bases, focusing on Arrhenius, Brønsted-Lowry, and Lewis theories. It explains the distinctions between strong and weak acids and bases, their ionization in aqueous solutions, and introduces the concept of conjugate acid-base pairs. Additionally, it highlights the limitations of the Arrhenius definition and the broader applicability of the Brønsted-Lowry theory in various chemical reactions.




























![Linkageofambidentateligandstometal atoms:
It is one of the important applications of the HSAB principle. The SCN-
ligand is an ambidentate
ligand and can be S-bound to metal (M-SCN) and referred to as thiocyanate or can be N-bound
to metal (M-NCS) and is referred to as isothiocyanate. The choice among S-bound or N-bound is
decided by soft or hard acid-base behavior. Sis a comparatively soft base than N atom. Hence soft
metal ions are S-bound while hard metal ions are N-bound.
1) SCN-
bonds through sulfuratom (soft base) when bonded to Pt2+
, a soft acid.
2) It bonds through nitrogen atom (a hard base) when linked to Cr3+
, a hard acid.
3)When Fe2+
reacts with SCN-
a bright red [Fe(SCN)]+
ion isformed, whereas Cr3+
forms [Cr(NCS)]2+
.
Reason: Fe2+
is a borderline acid and is S-bound. Whereas Cr3+
is hard acid and prefers to be N-
bound.
4)The molecule (CH3)2NCH2PF2 would bond to BF3through N whereas itwould bond to BH3through
P.
Reason: BF3is a hard acid and prefers to bind with N atom - a hard base. Whereas, BH3 is a soft
acid and preferentially bonded to soft base, P atom.](https://image.slidesharecdn.com/acid20base20concepts-210316124213/75/Acid-base-concepts-Acid-Base-concepts-Arrhenius-Lowery-Bronsted-Lewis-Soft-and-hard-acid-base-concept-Applications-of-SHAB-52-2048.jpg)
![Symbioticeffect:
□ The hard-soft character of the metal ion is altered by the other groups attached. It is
referred to as a symbioticeffect.
□ For example, the isolated Co3+
is a hard acid and is expected to make the bond with
SCN-
ion through N atom as observed in[Co(NH3)5(NCS)]3-
.
□ However, when bound to five soft base ligands like CN-
ions, the hardness of cobalt
ion (Co3+
)is reduced. Thus [Co(CN)5]2-
behaves as a soft acid and prefers to bind with
SCN-
ion through Satom to form [Co(CN)5(SCN)]3-
.](https://image.slidesharecdn.com/acid20base20concepts-210316124213/75/Acid-base-concepts-Acid-Base-concepts-Arrhenius-Lowery-Bronsted-Lewis-Soft-and-hard-acid-base-concept-Applications-of-SHAB-53-2048.jpg)