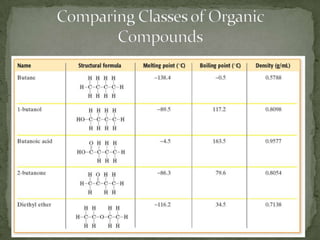
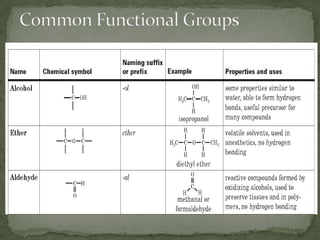
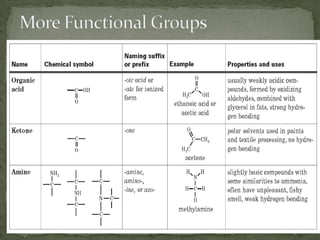
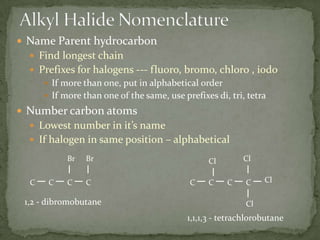
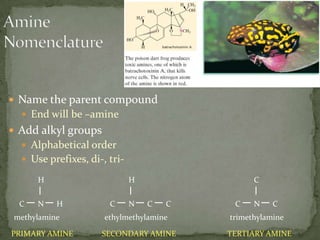
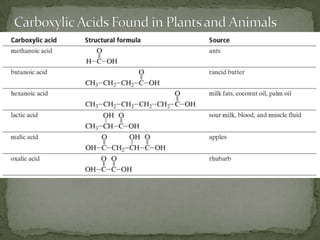
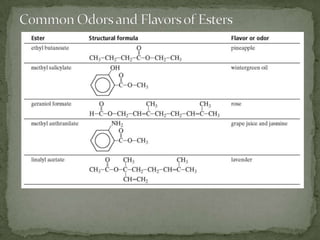
This document defines functional groups and discusses various classes of organic compounds organized by their functional groups, including alcohols, alkyl halides, ethers, aldehydes, ketones, carboxylic acids, esters, and amines. It provides the general formulas and properties of each class and describes their naming conventions based on the functional group present. Key points covered include that functional groups determine properties, common uses of each class, and relationships between structure and reactivity.