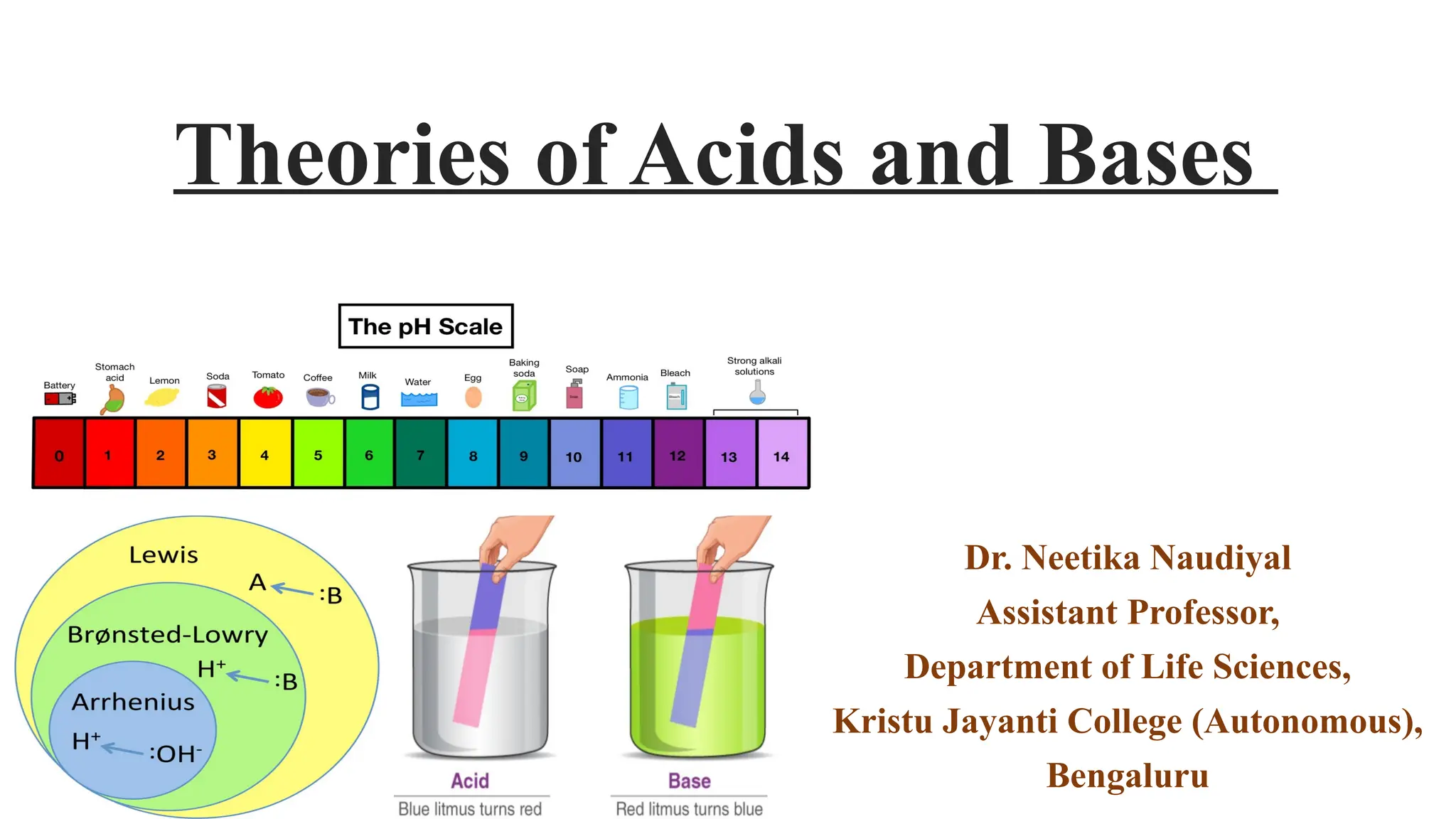
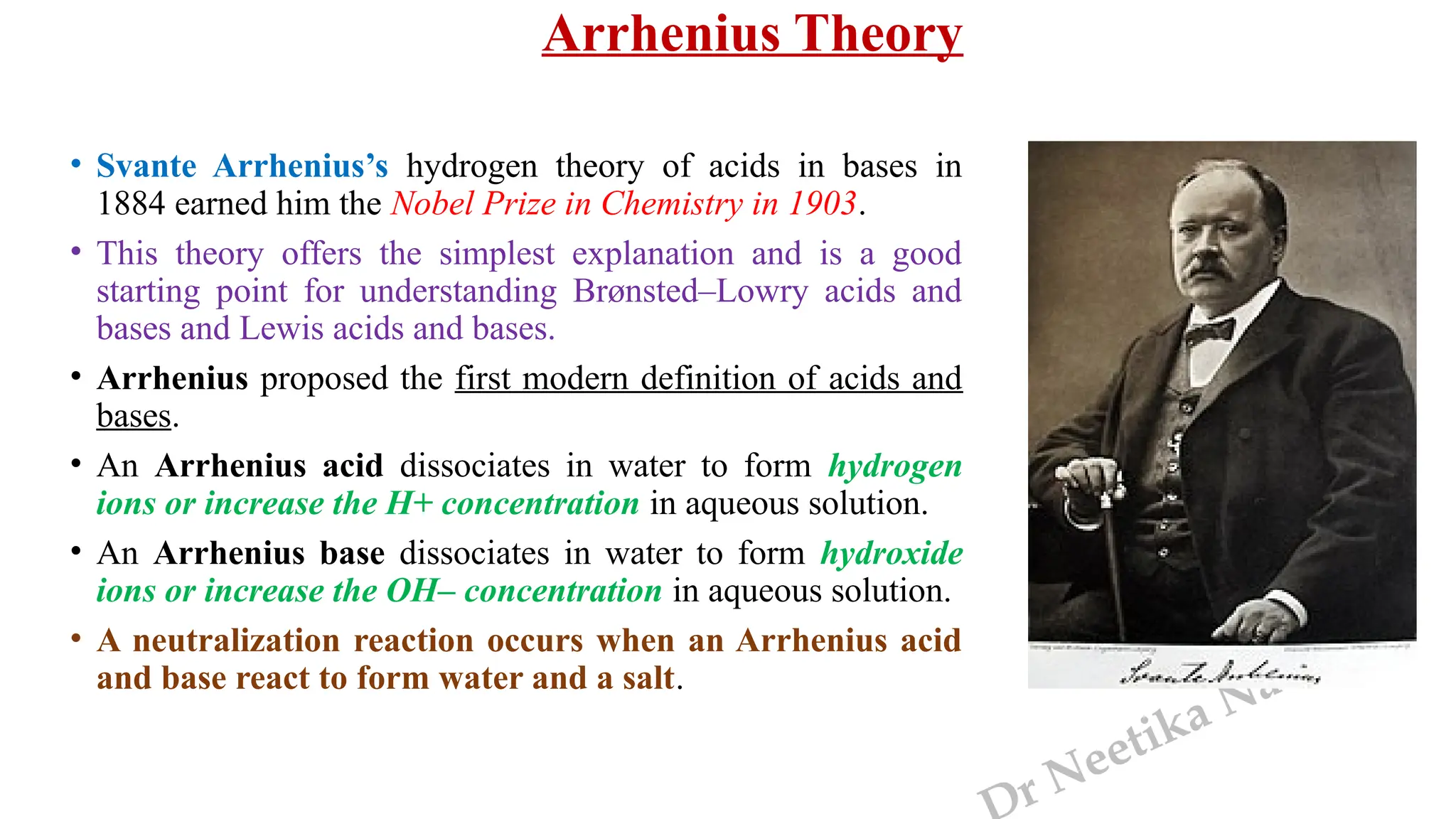
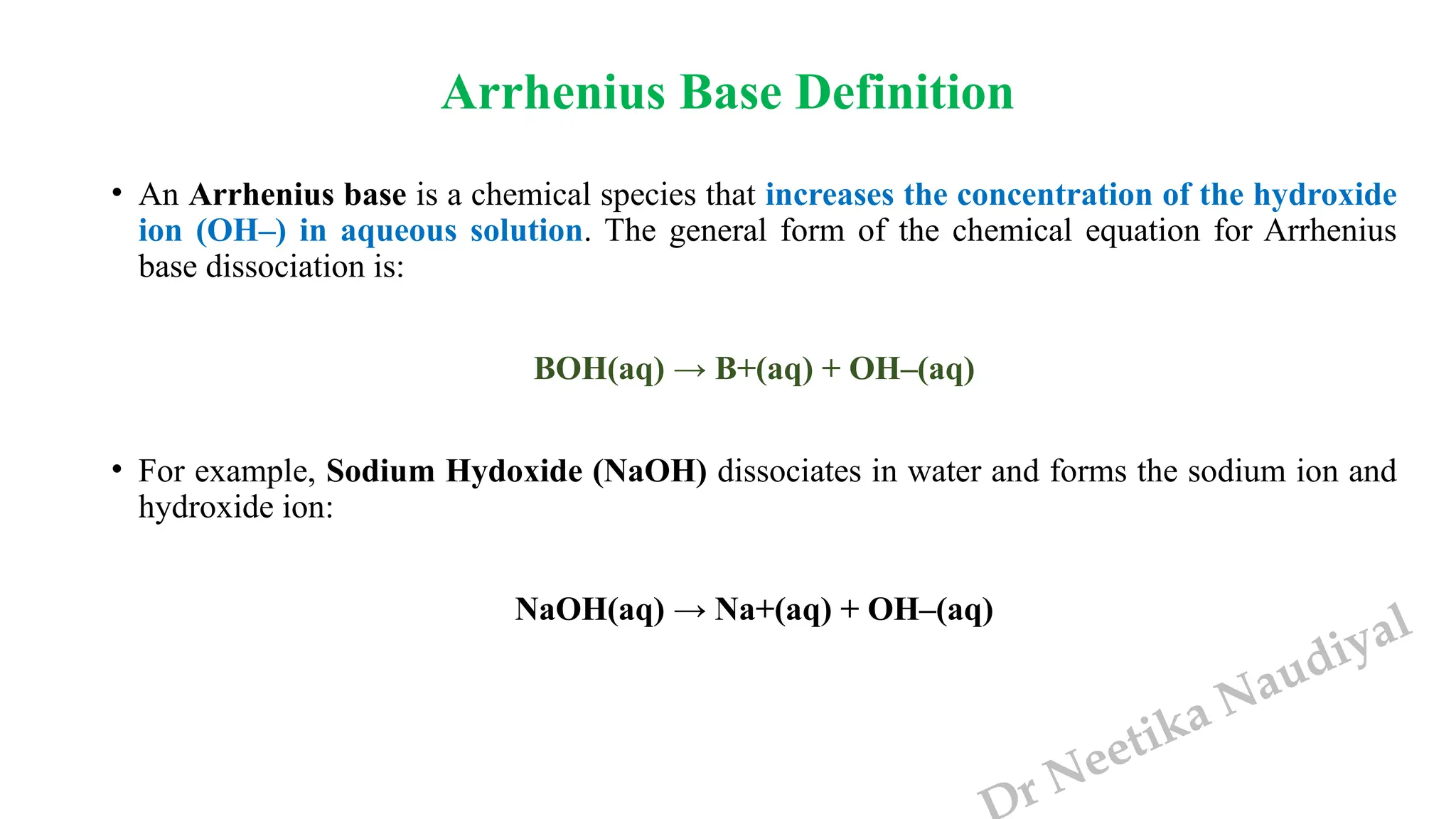
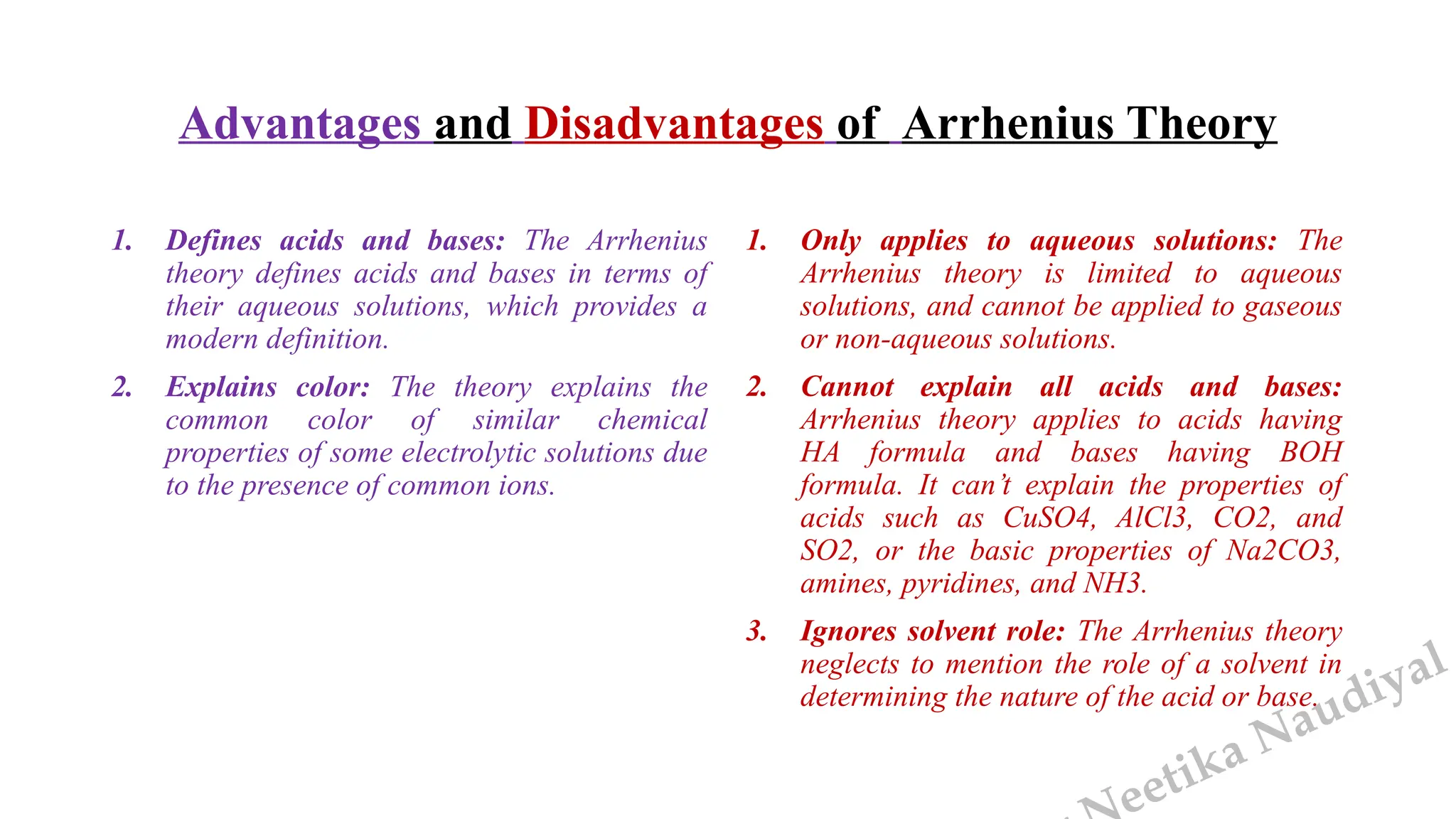
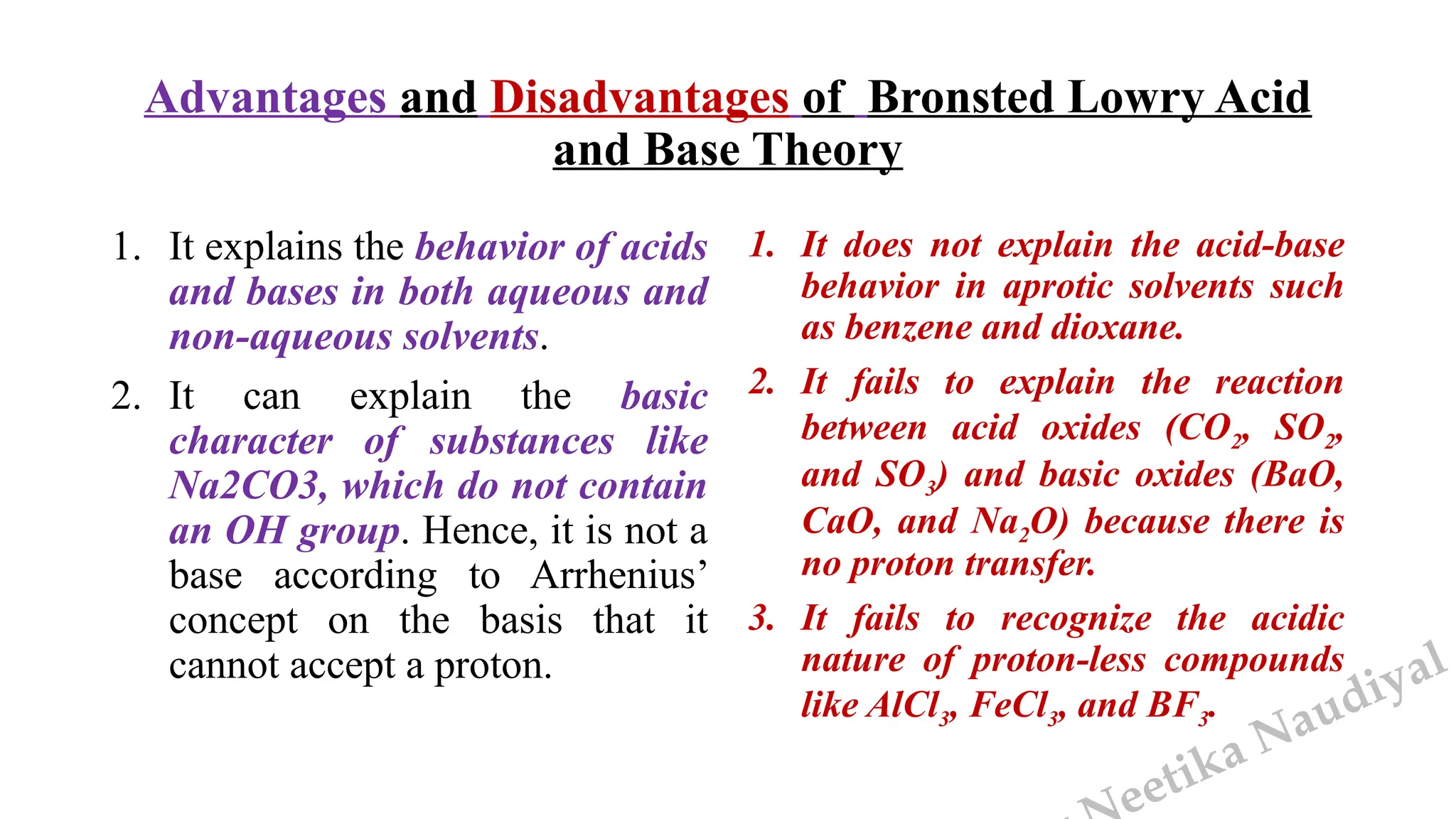
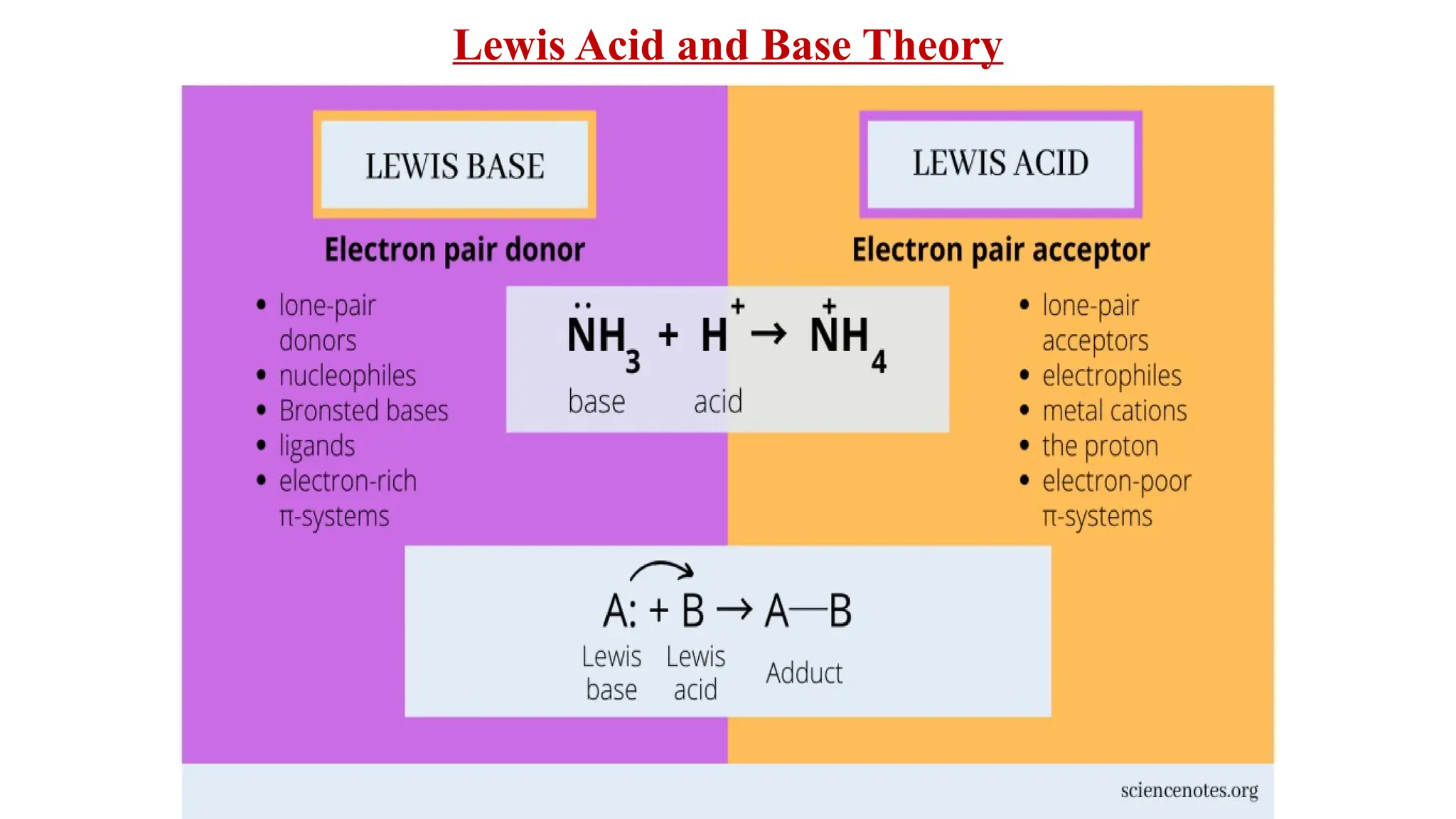
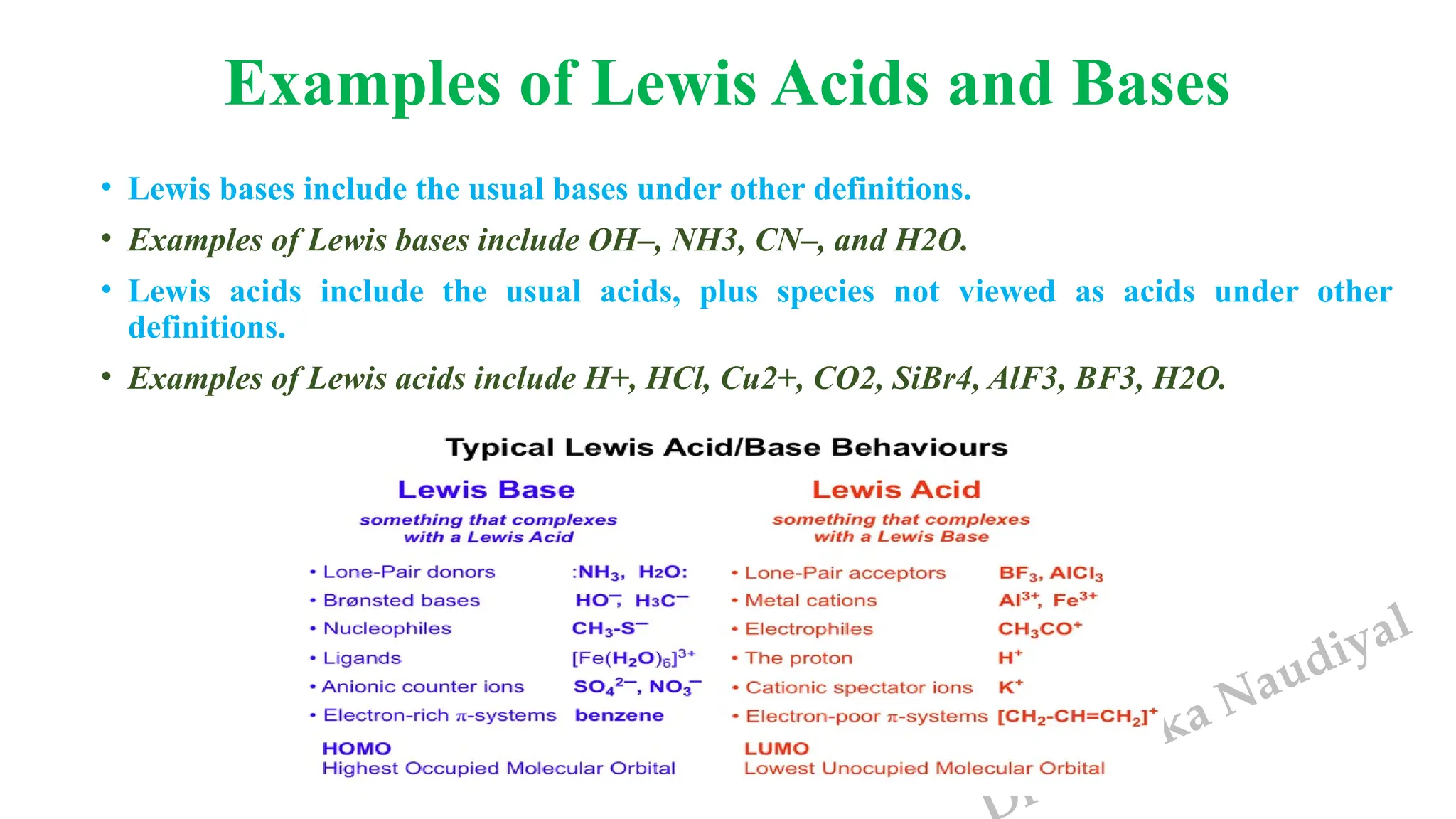
The document presents the three main theories of acids and bases: the Arrhenius, Brønsted-Lowry, and Lewis theories. Each theory offers distinct definitions, advantages, and limitations regarding the behavior of acids and bases in various contexts, including their reactions in aqueous and non-aqueous solutions. Examples and mechanisms of acid-base reactions, as well as identifying strong and weak acids and bases, are thoroughly discussed throughout the text.











![r Neetika Naudiyal
Amphoteric Species
• Some chemical species are amphoteric, meaning they can act as either a Lewis acid or as a Lewis base,
depending on the situation.
• Water (H2O) is a great example.
Water acts as an acid when it reacts with ammonia:
H2O + NH3 → NH4+ + OH−
It acts as a base when it reacts with hydrochloric acid:
H2O + HCl → Cl– + H3O+
• Aluminum hydroxide [Al(OH)3] is an example of an amphoteric compound under the Lewis theory.
It acts as a Lewis base in the reaction with the hydrogen ion:
Al(OH)3 + 3H+ → Al3+ + 3H2O
It acts as a Lewis acid in the reaction with the hydroxide ion:
Al(OH)3 + OH− → Al(OH)4–](https://image.slidesharecdn.com/theoriesofacidsandbases-241121095300-46909cbf/75/Theories-of-Acids-and-Bases-Arrhenius-Bronsted-and-Lewis-19-2048.jpg)

