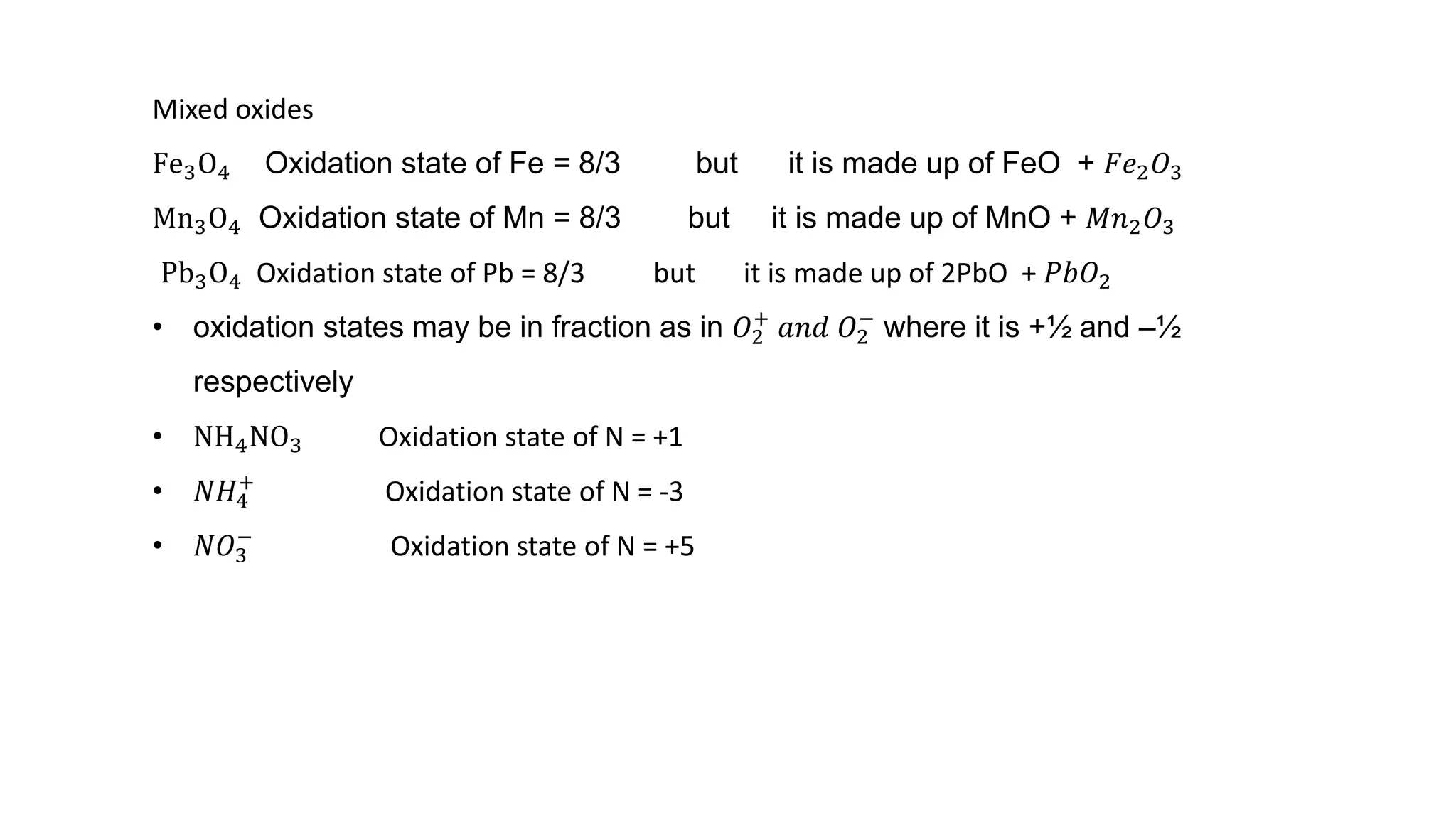
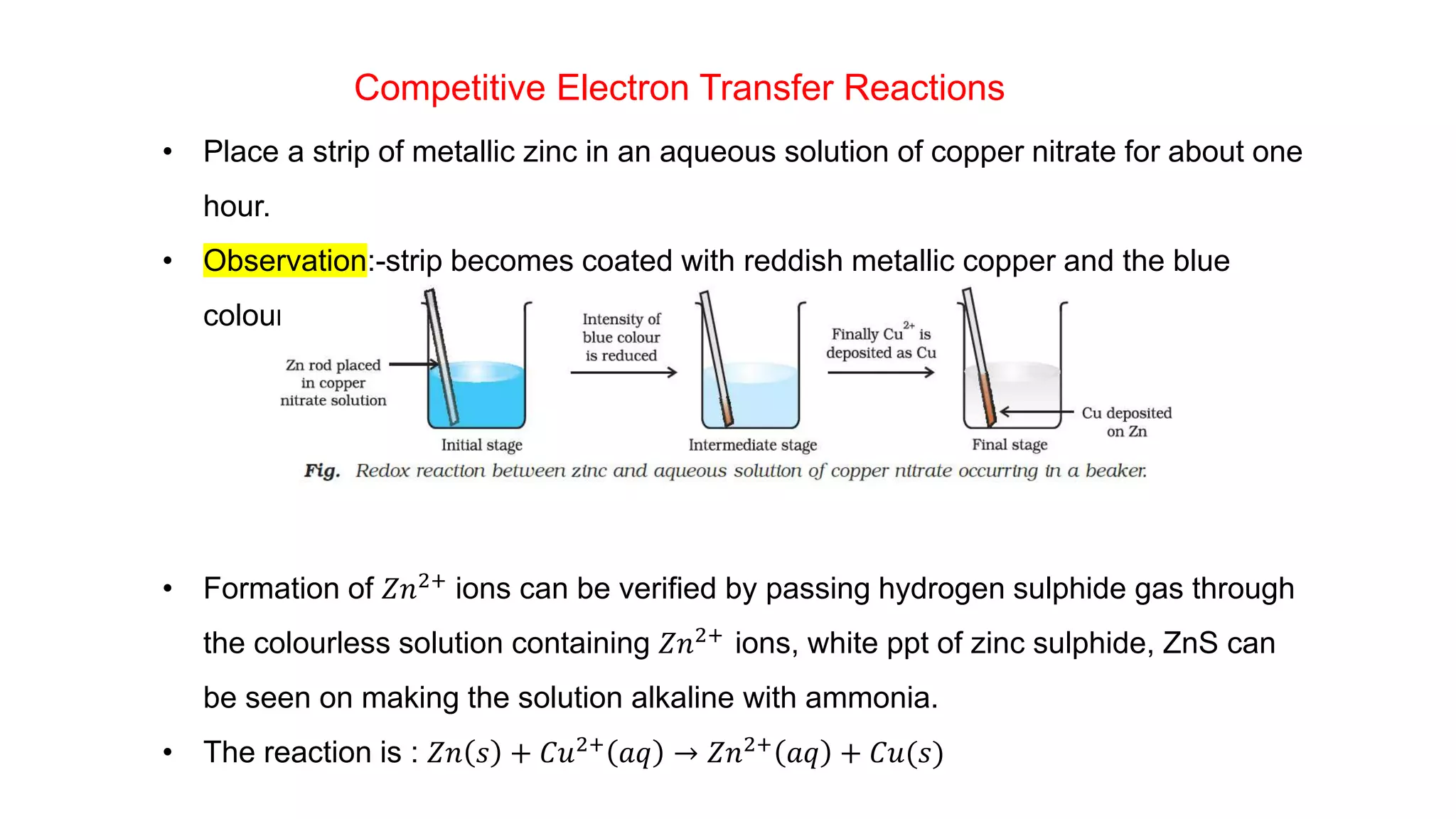
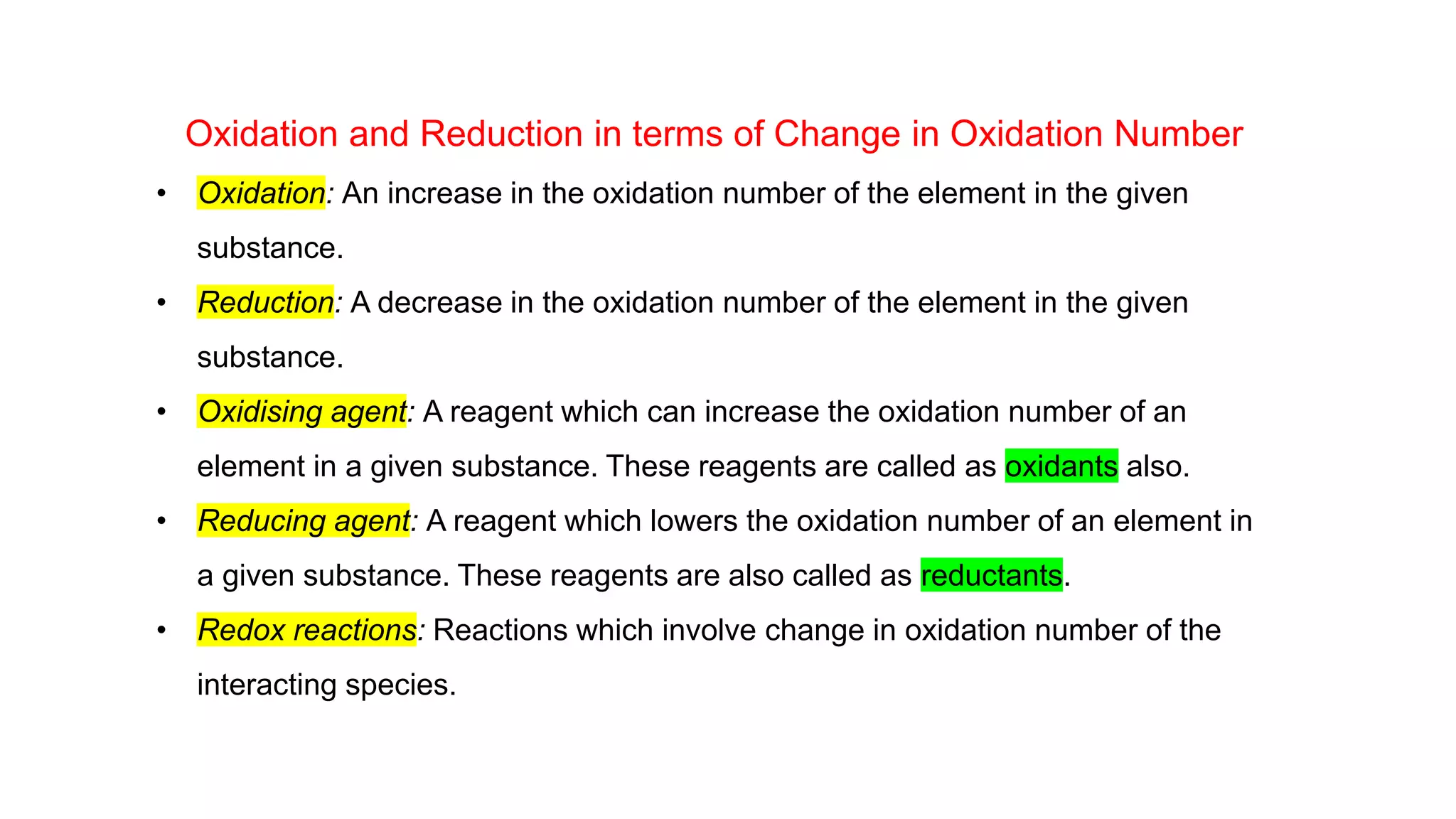
1) The document discusses classical ideas of oxidation and reduction reactions by defining them as addition or removal of oxygen, hydrogen, or electronegative/electropositive elements.
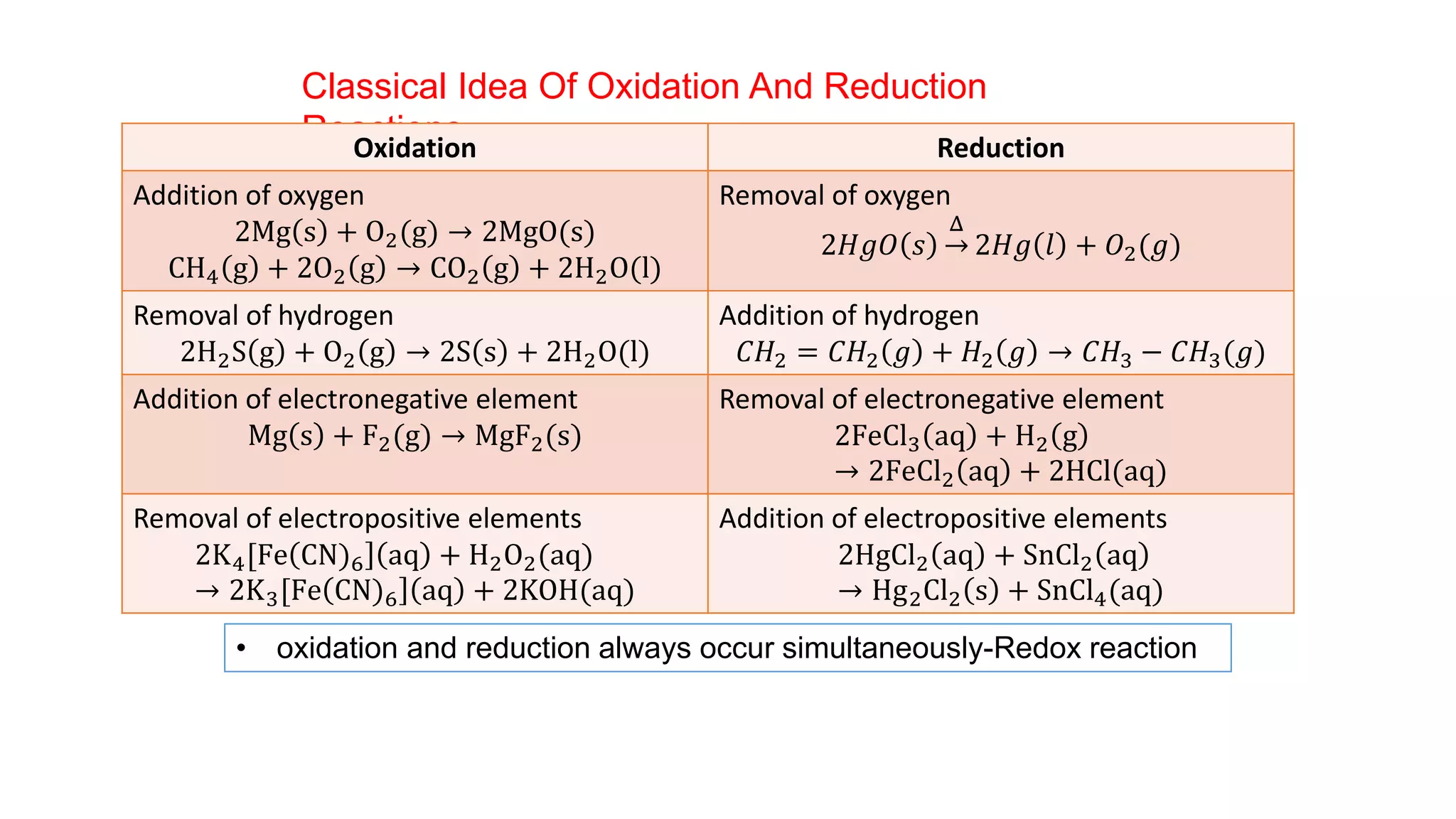
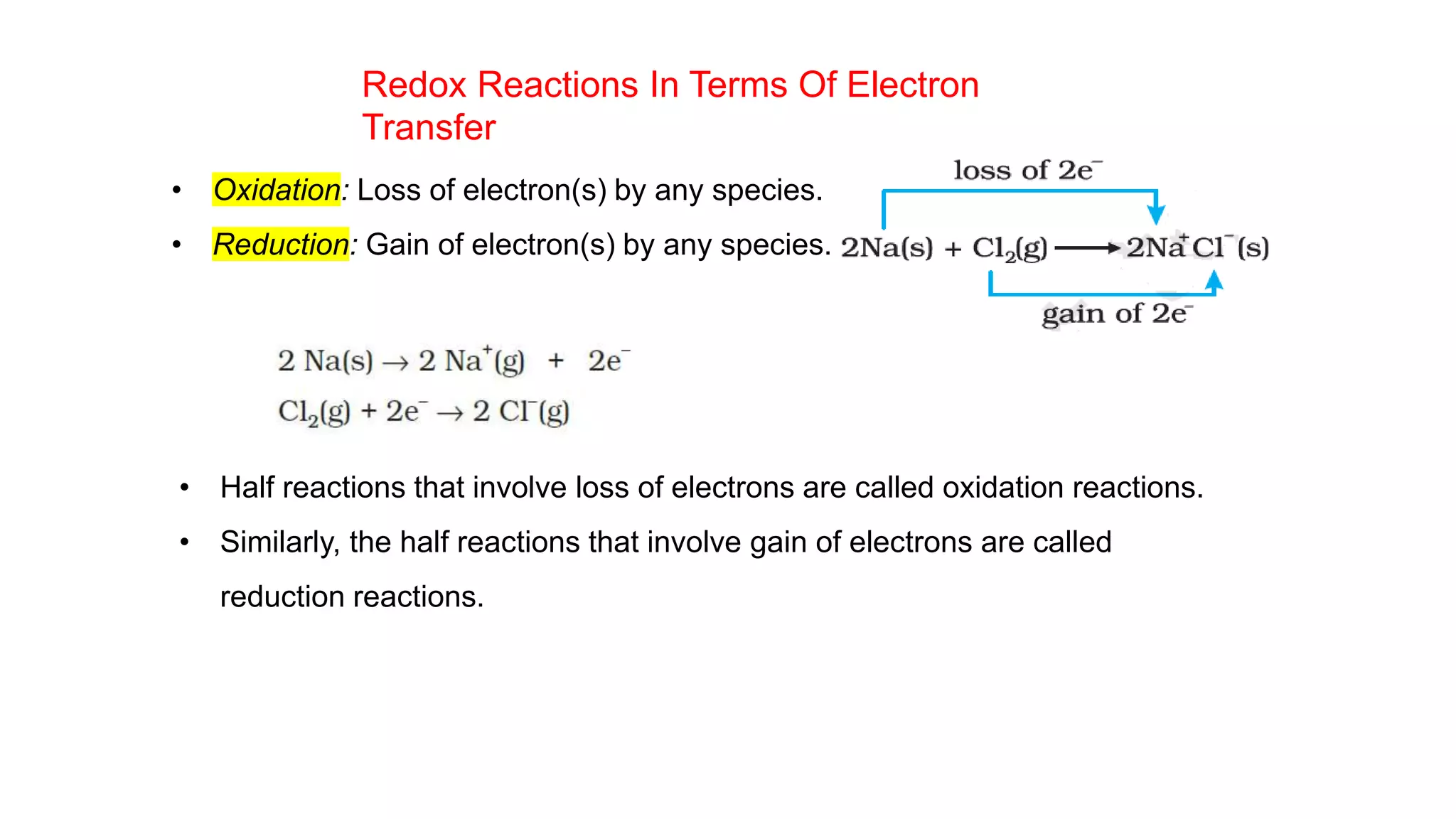
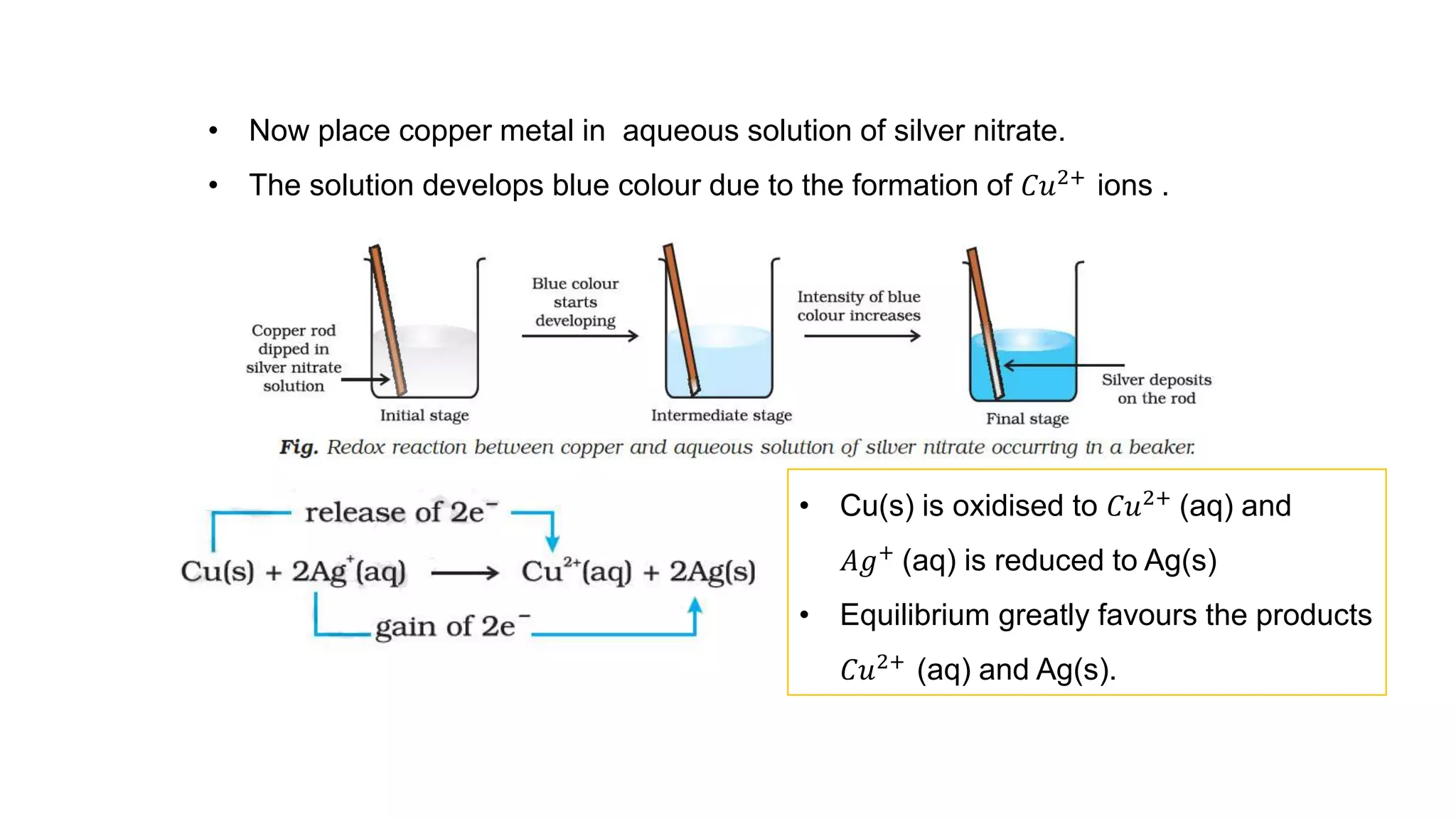
2) It then moves to discussing redox reactions in terms of electron transfer, defining oxidation as loss of electrons and reduction as gain of electrons.
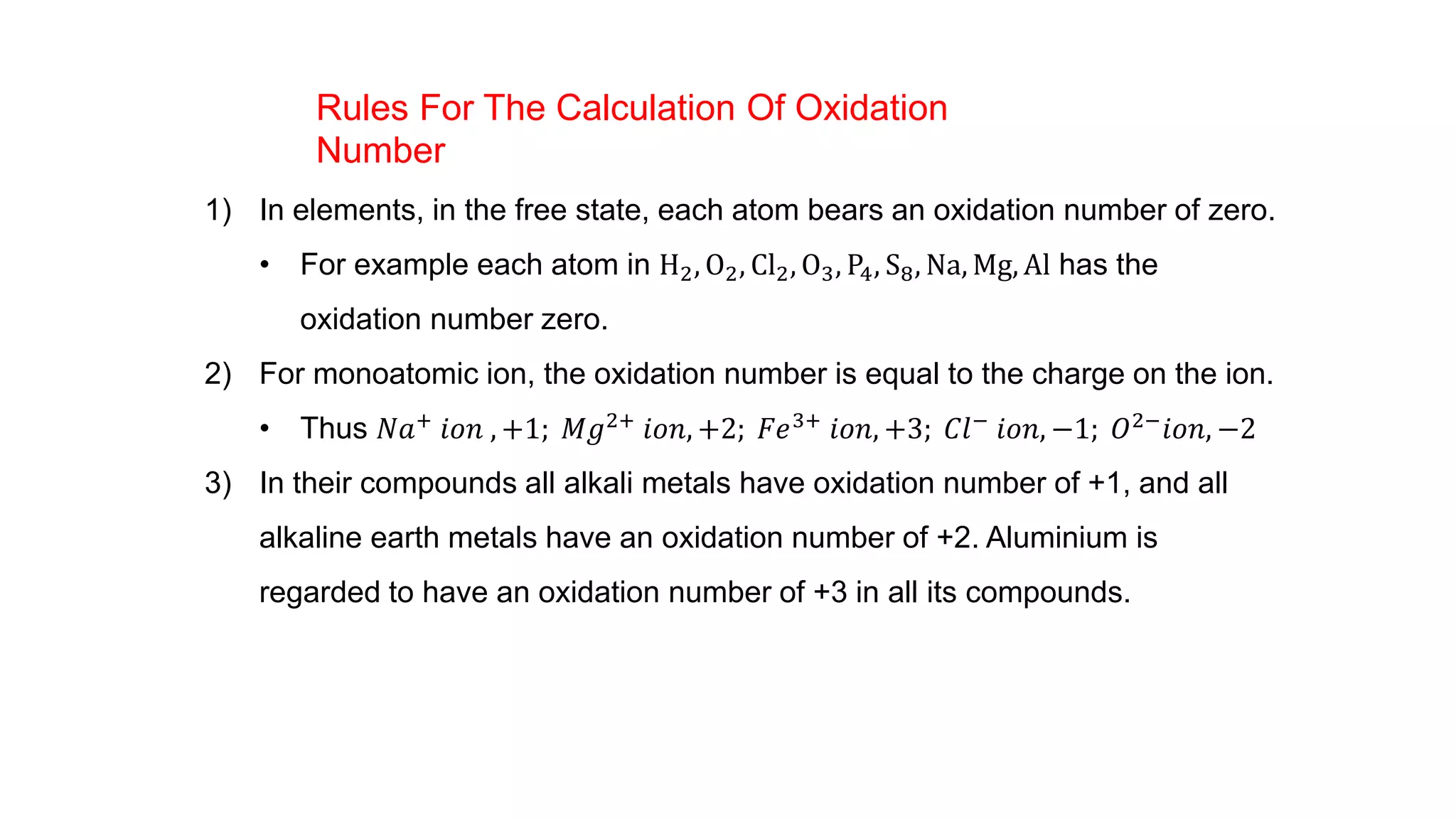
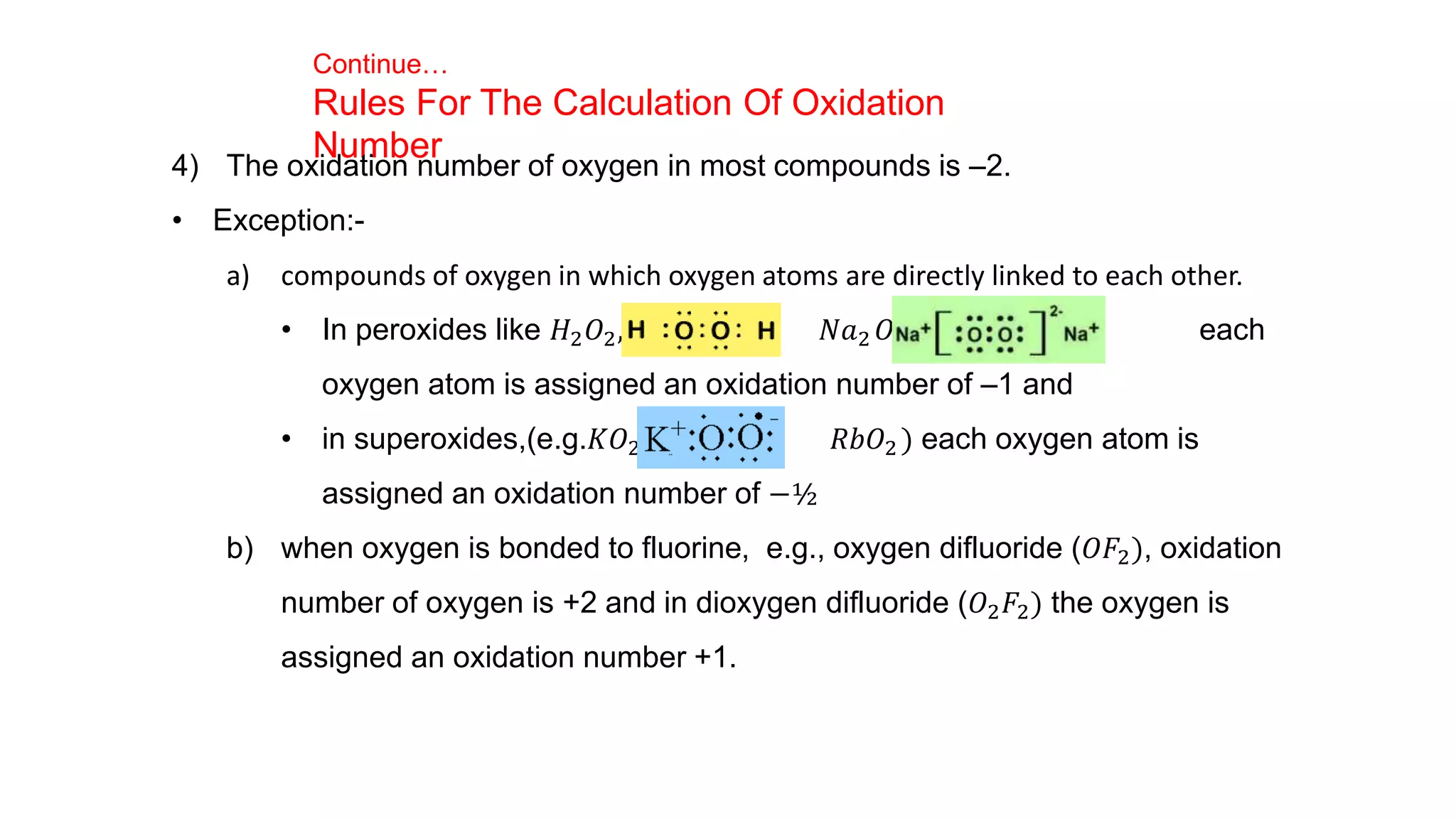
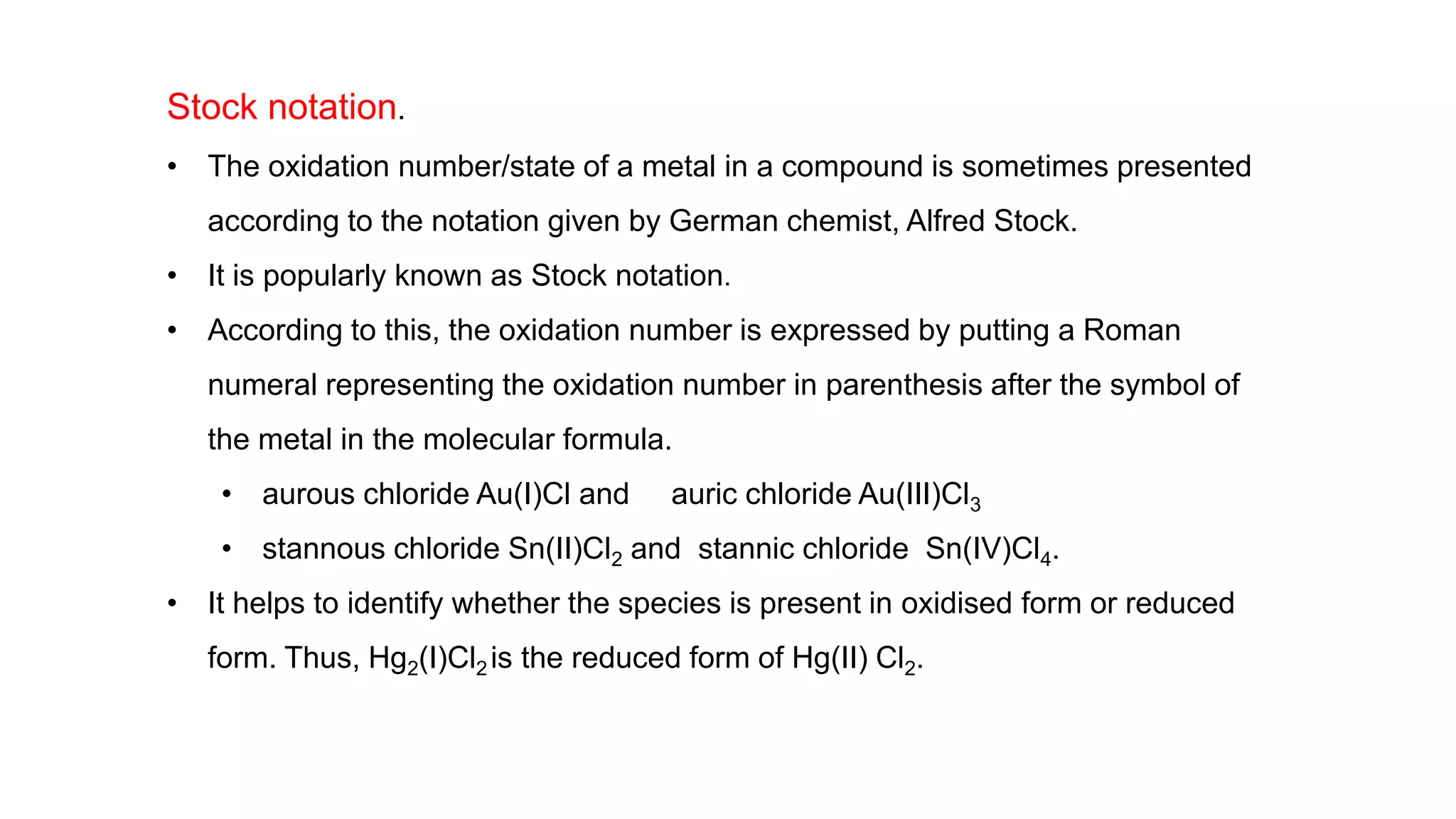
3) Rules for calculating oxidation numbers are provided, including that the sum of oxidation numbers in a compound or ion must equal the overall charge. Stock notation is also introduced for representing oxidation states.
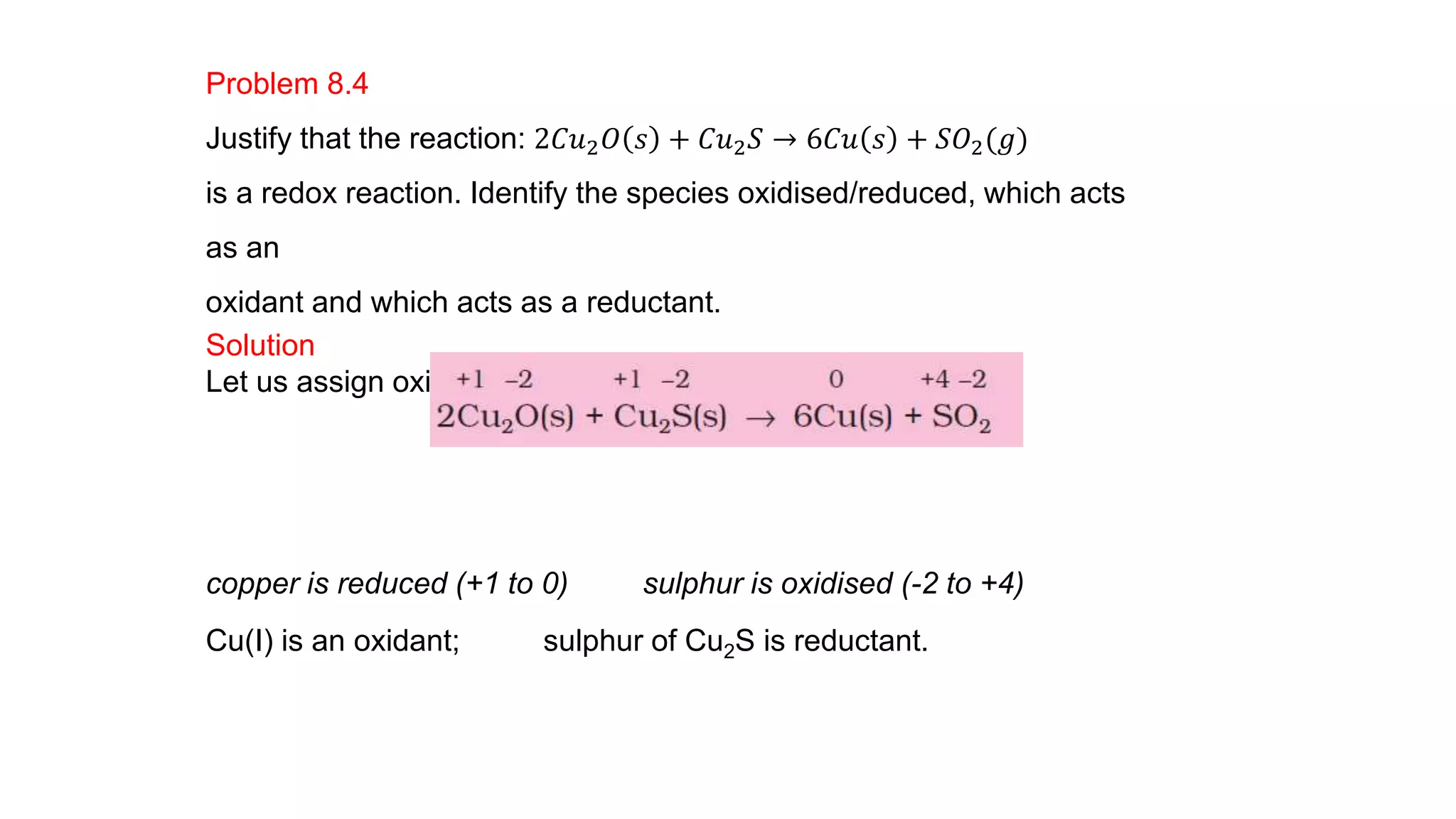
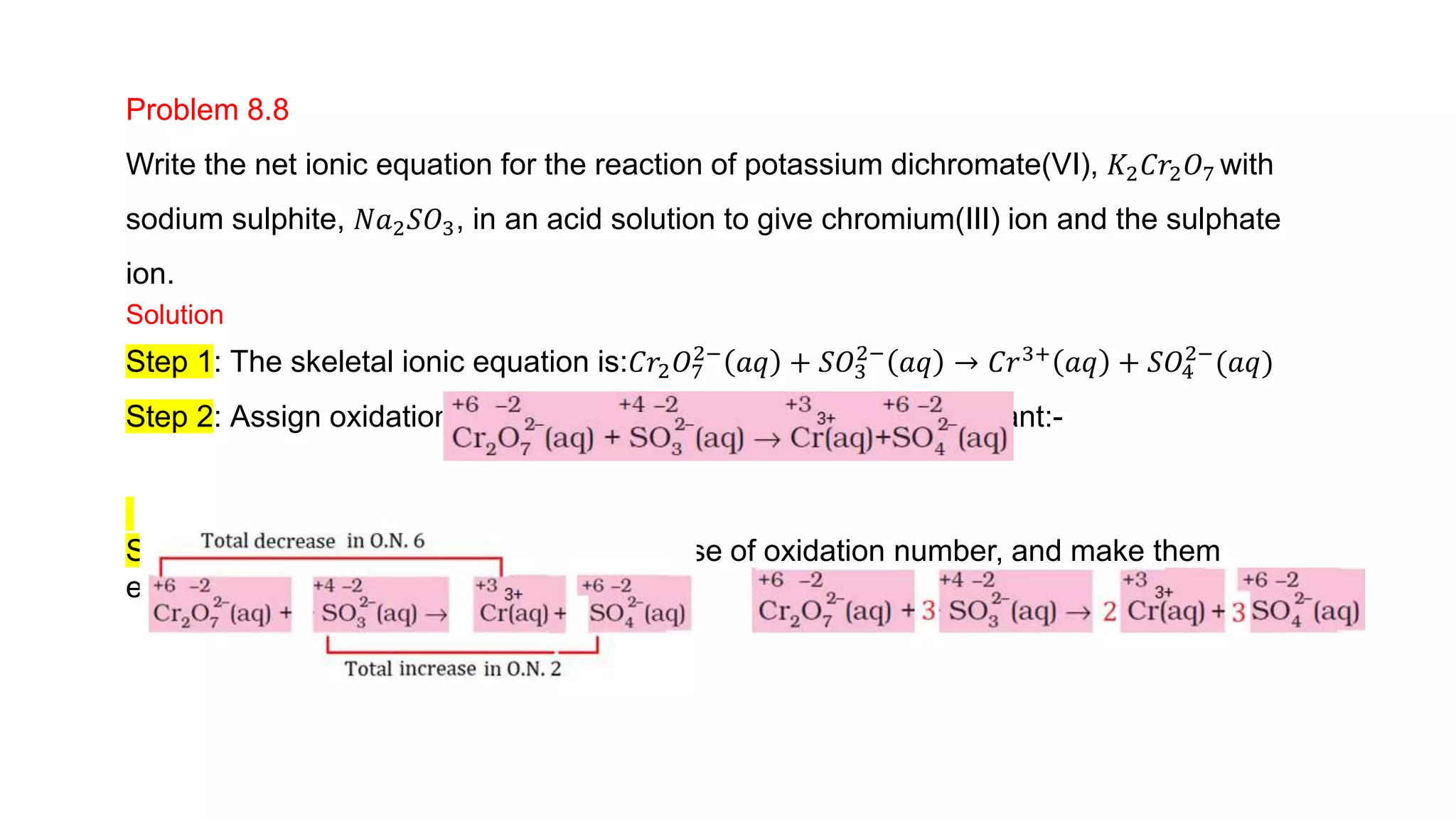
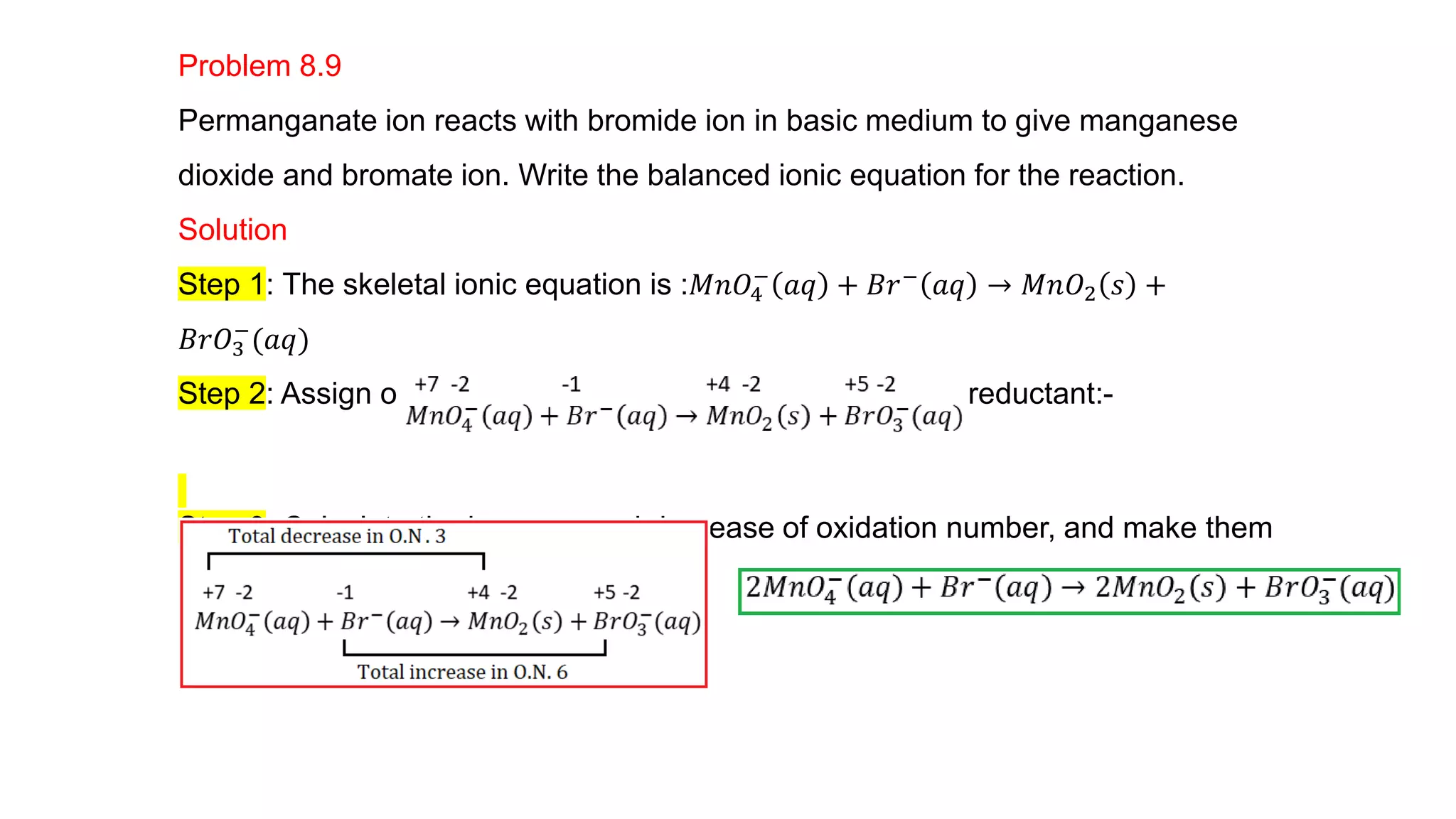
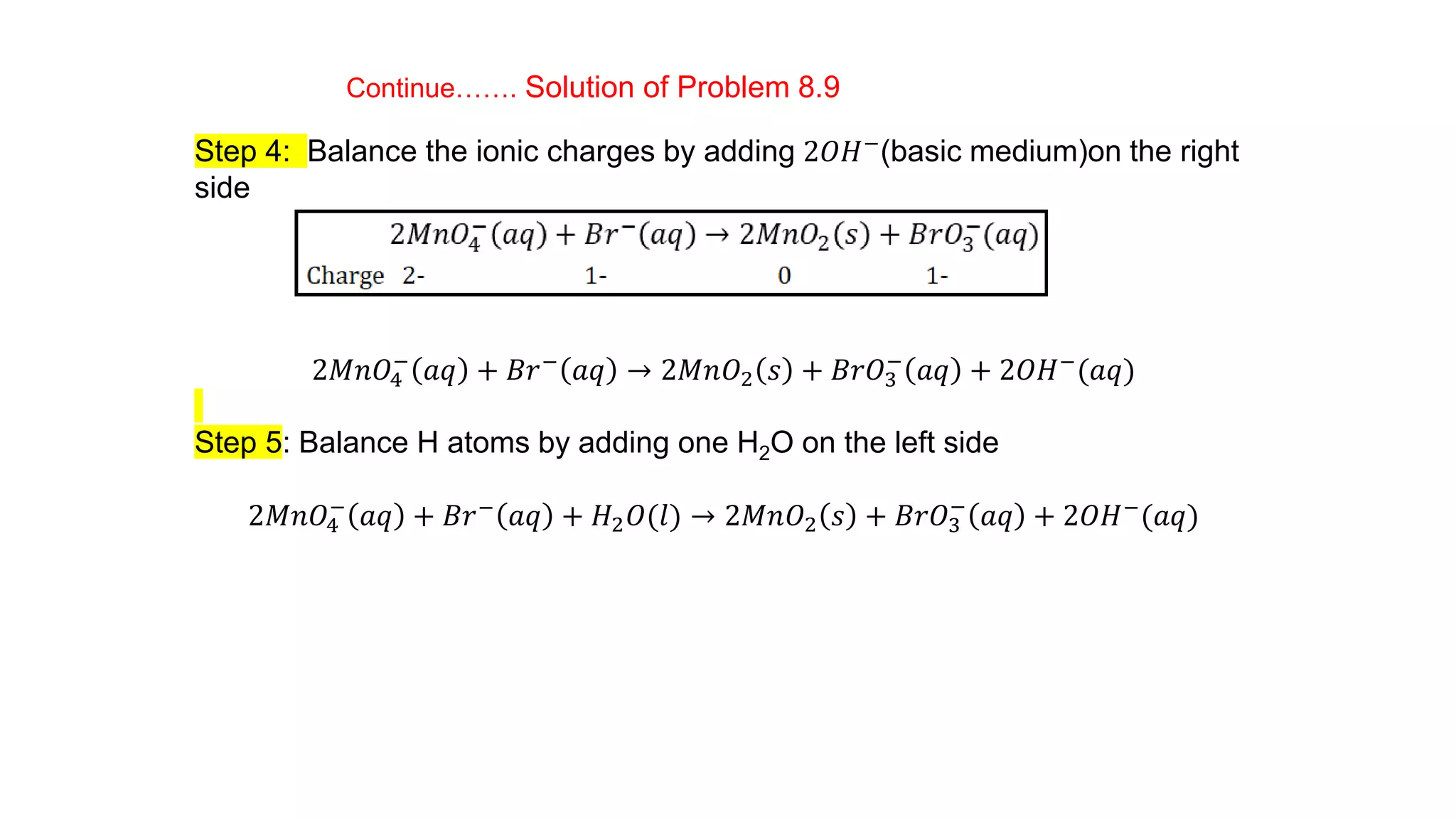
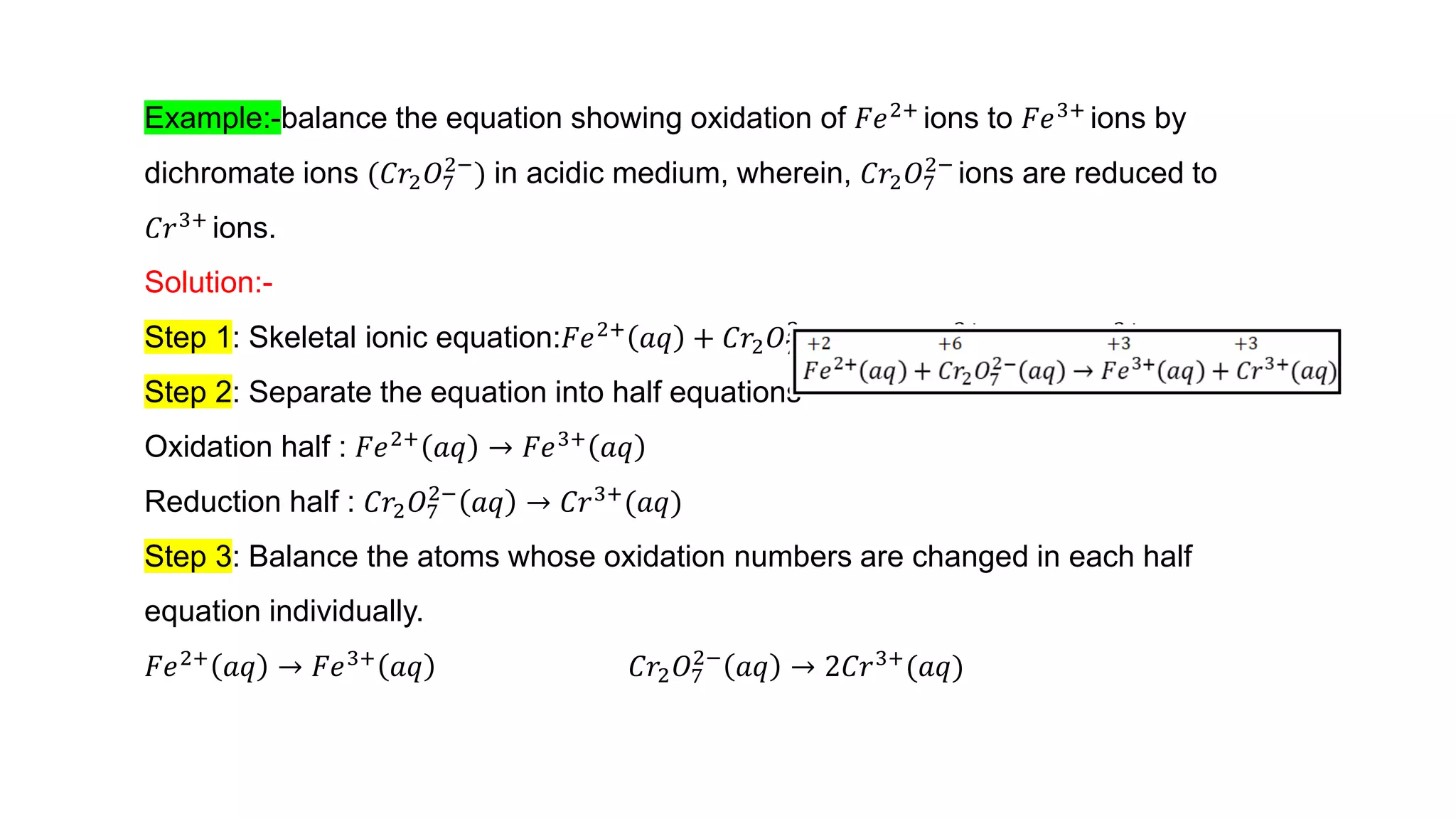
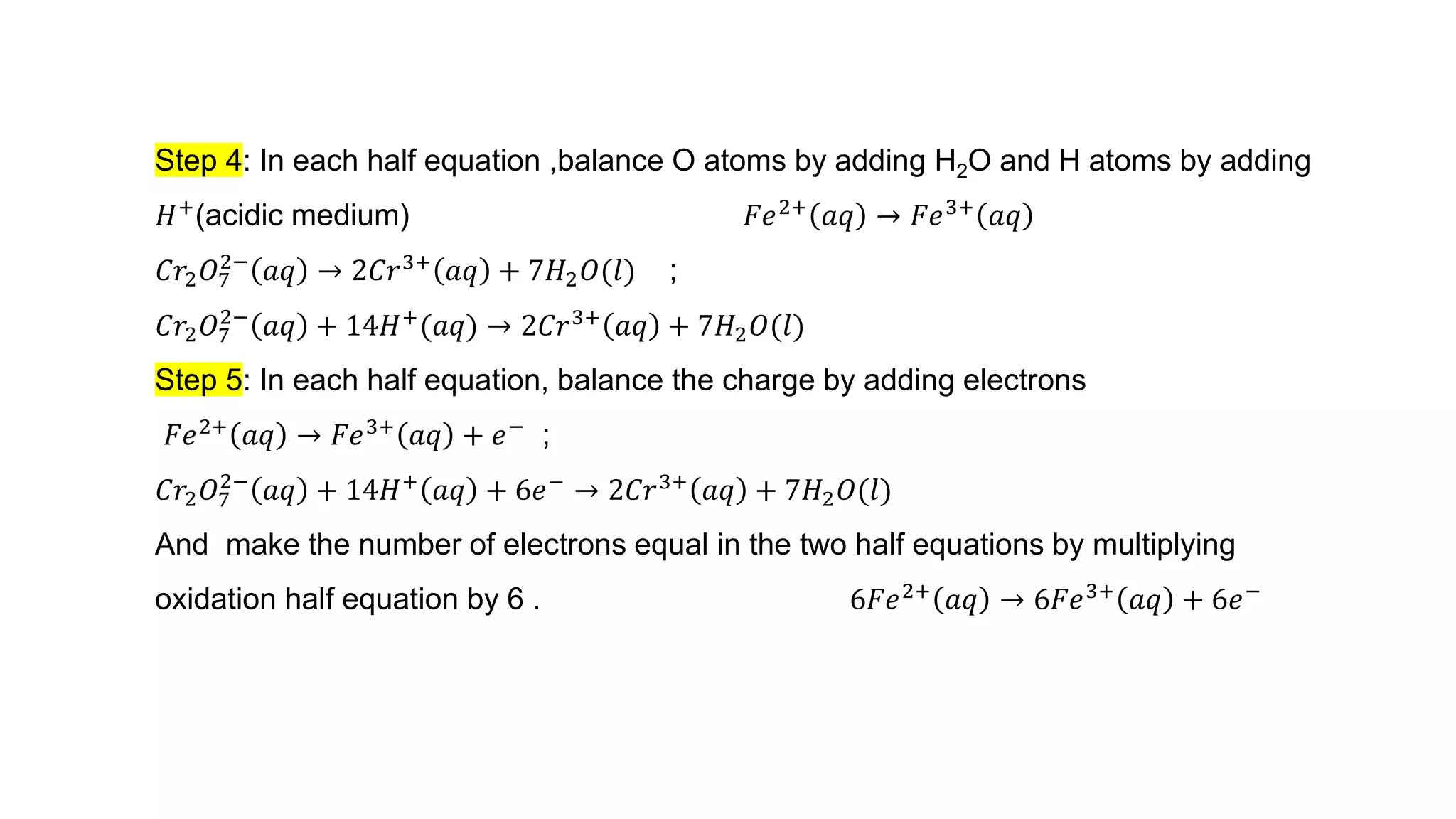
4) Examples are given of identifying oxidizing and reducing agents, balancing redox reactions using the oxidation number method, and classifying reactions as redox based on changes in oxidation numbers.



































![The Paradox of Fractional Oxidation
Number
A paradox (विरोधाभास)is a statement in which it seems that if one part of it
is true, the other part of it cannot be true.
• Sometimes, we come across with certain compounds in which the oxidation number
of a particular element in the compound is in fraction.
• Examples are:
• 𝐶3𝑂2 Carbon suboxide [where oxidation number of carbon is (4/3)],
• 𝐵𝑟3𝑂8 tribromooctaoxide [where oxidation number of bromine is (16/3)]
• 𝑁𝑎2𝑆4𝑂6 Sodium Tetrathionate (where oxidation number of sulphur is 2.5).](https://image.slidesharecdn.com/class11chapter8redoxreactions-230213165058-65aba2a8/75/Class-11-Chapter-8-Redox-Reactions-pptx-55-2048.jpg)