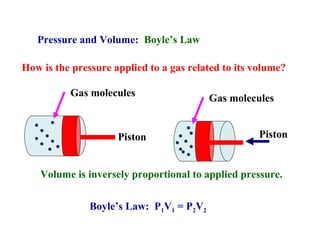
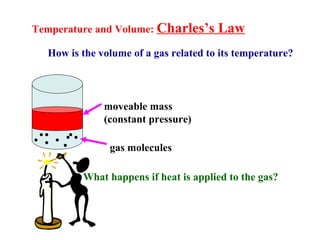
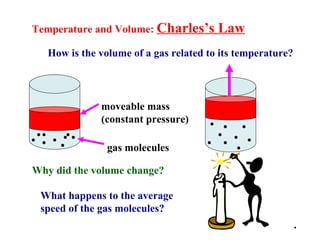
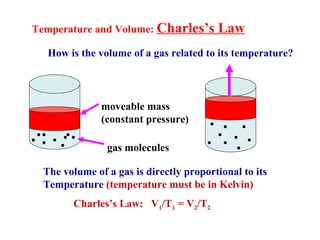
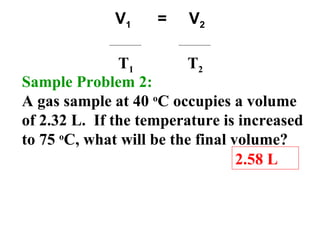
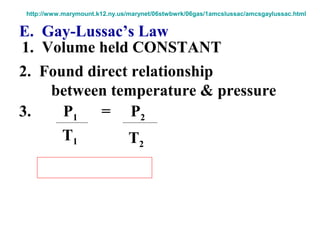
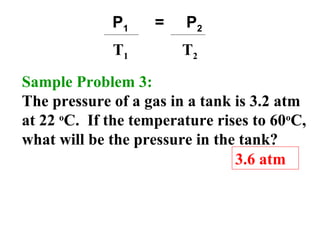
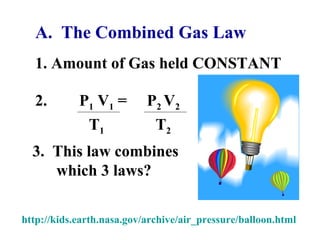
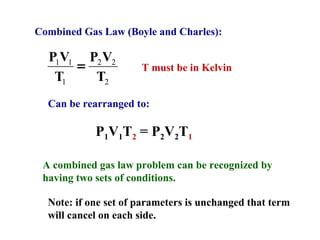
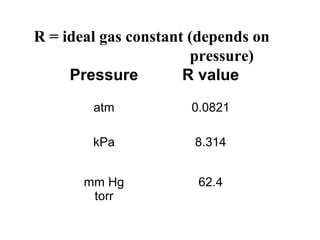
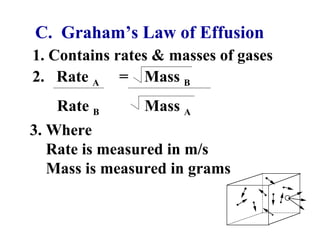
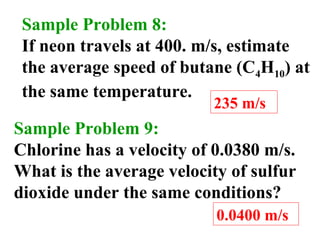
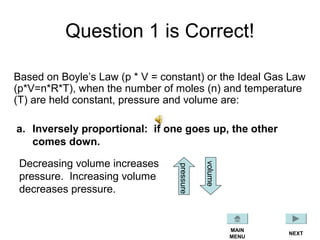
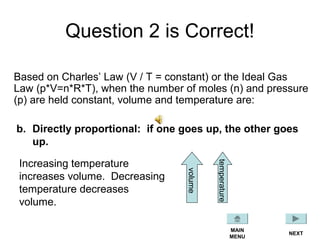
This document discusses the key gas laws and their relationships. It begins by introducing the four main properties that determine gas behavior: pressure, volume, amount of gas, and temperature. It then explains each gas law in more detail: Boyle's Law describes the inverse relationship between pressure and volume; Charles' Law specifies the direct relationship between volume and temperature; Gay-Lussac's Law shows the direct link between pressure and temperature; and the Combined Gas Law incorporates all three. The document also presents sample problems demonstrating applications of the gas laws.