Embed presentation
Downloaded 32 times







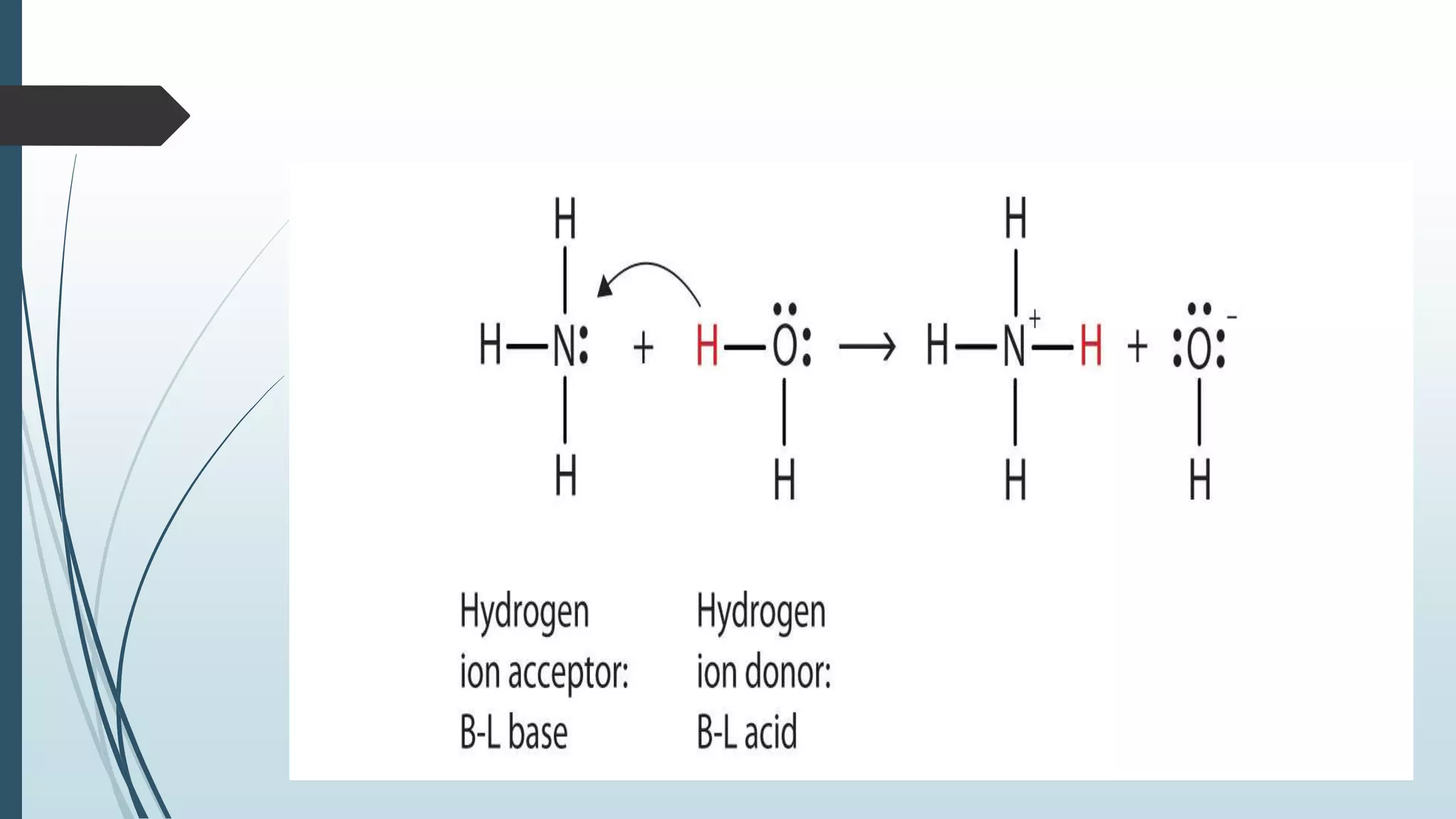


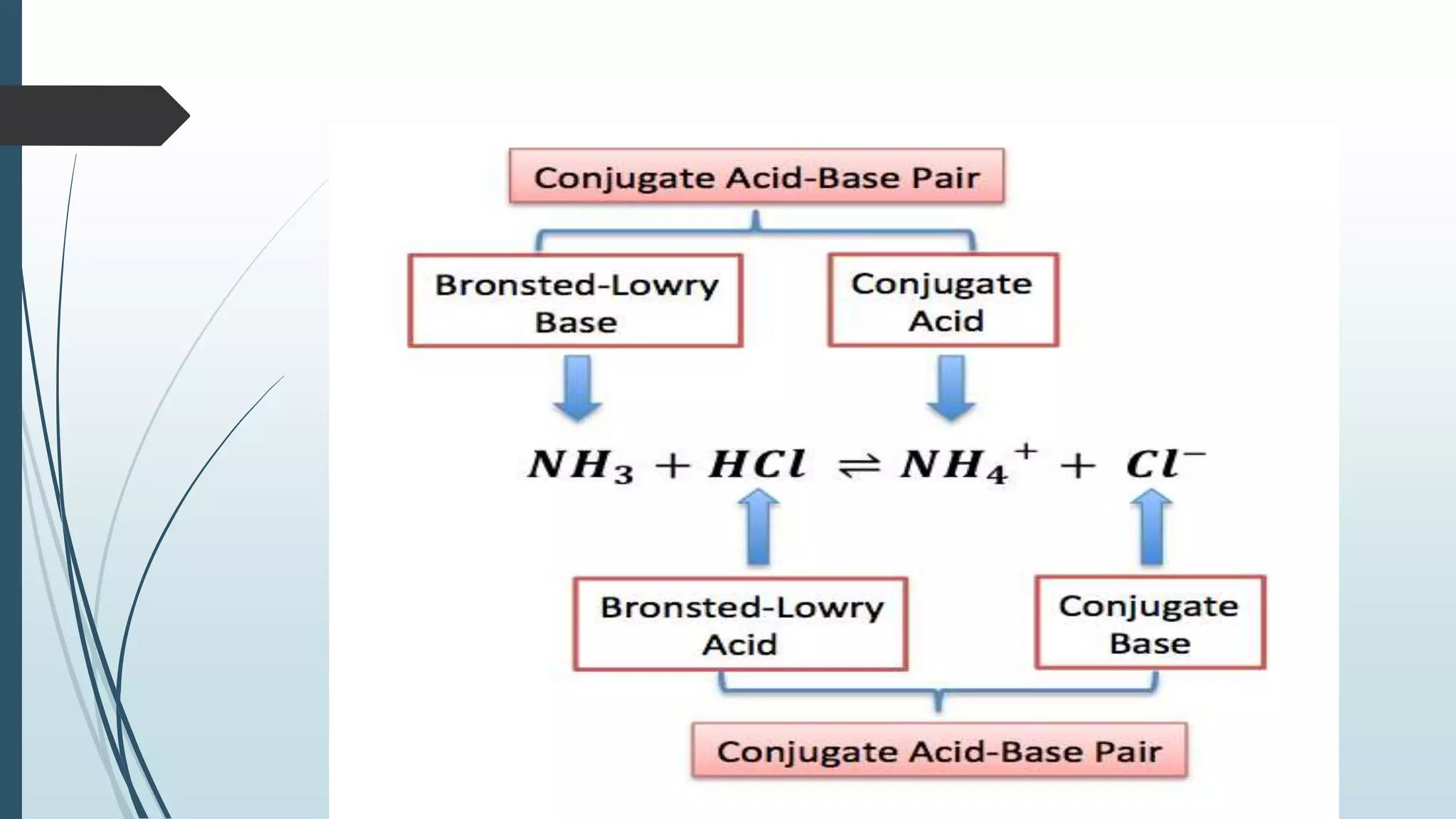


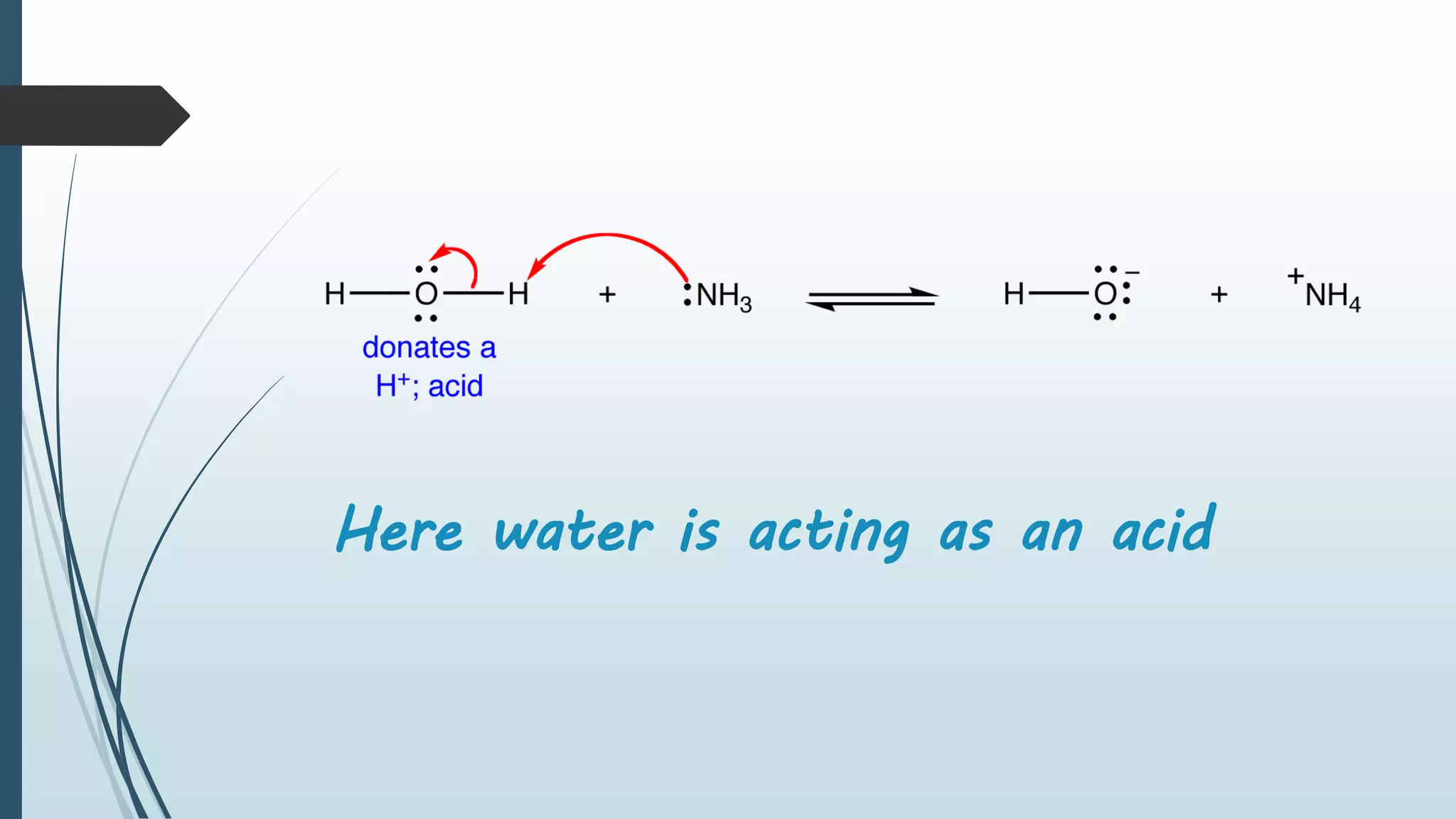


Bronsted and Lowry introduced the concept of acids and bases in 1923. According to Bronsted-Lowry theory, an acid is a proton donor and can donate a hydrogen ion to another molecule, while a base is a proton acceptor and can accept a hydrogen ion from another molecule. The conjugate acid of a base is the molecule formed when the base accepts a proton, while the conjugate base of an acid is the molecule formed when the acid donates a proton. Water is provided as an example of an amphoteric substance that can act as both an acid and a base by donating or accepting protons.















