This document summarizes key concepts about atomic structure:
1) It describes early atomic models including Democritus' idea of indivisible atoms and Dalton's atomic theory.
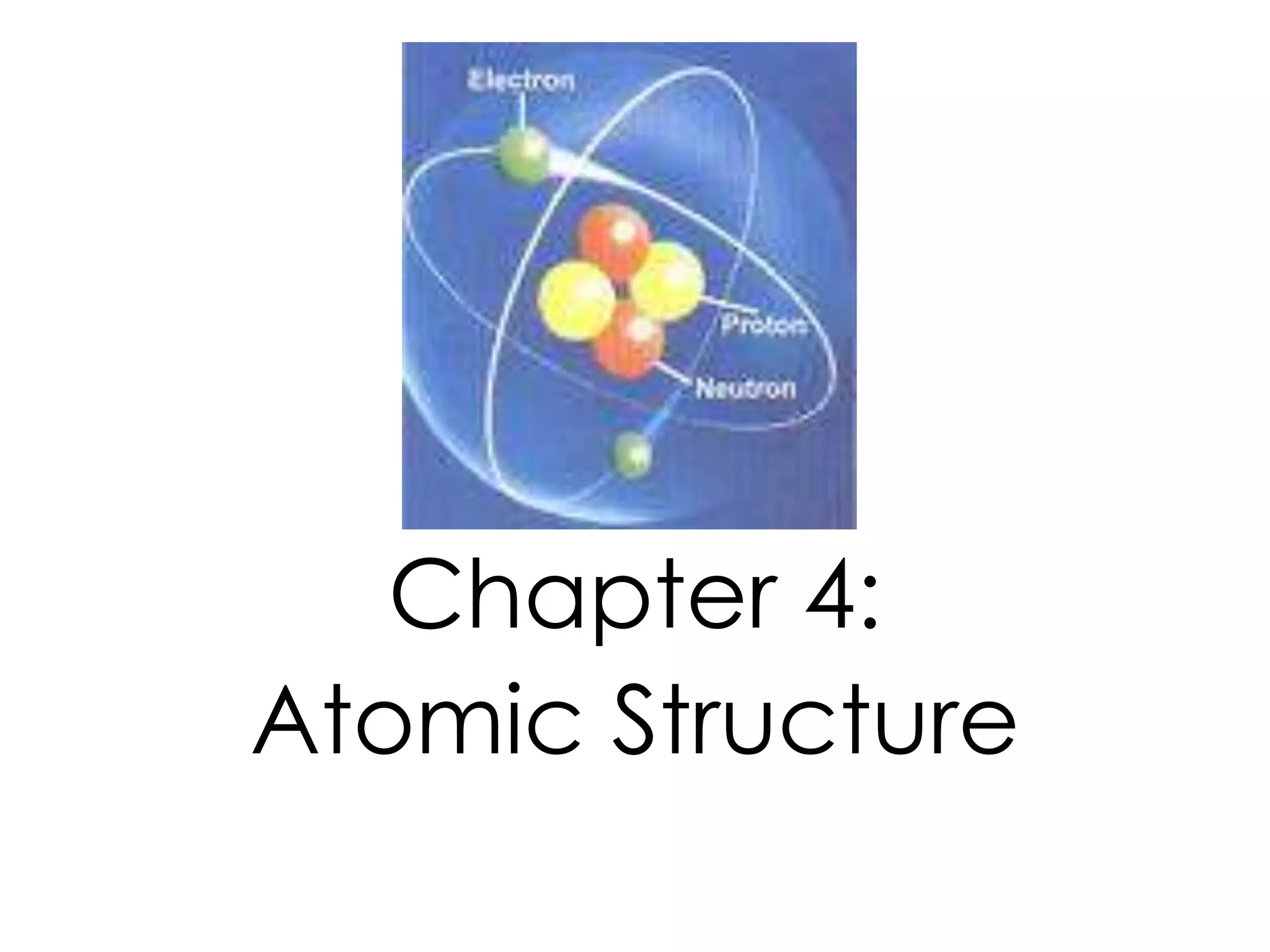
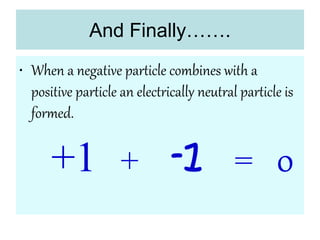
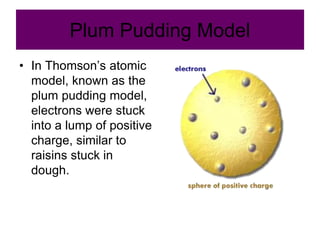
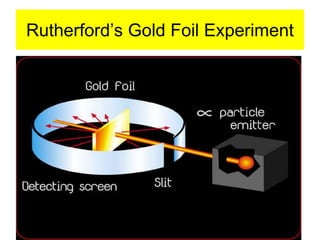
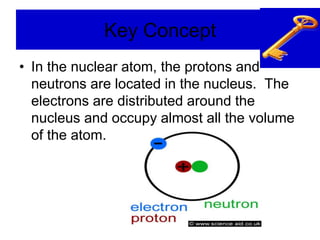
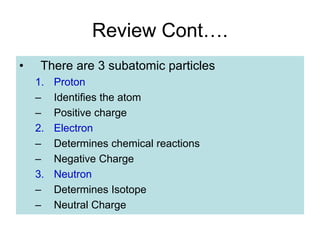
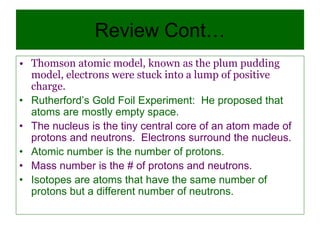
2) It explains the discovery of subatomic particles (electrons, protons, neutrons) and the nuclear model of the atom with electrons orbiting a nucleus.
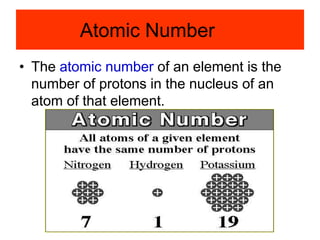
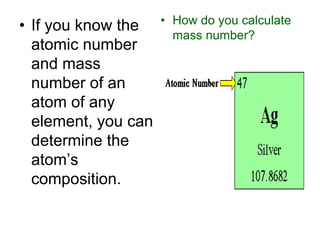
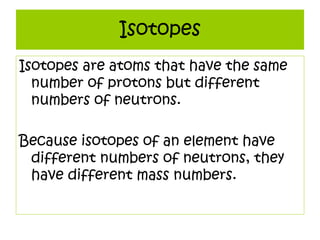
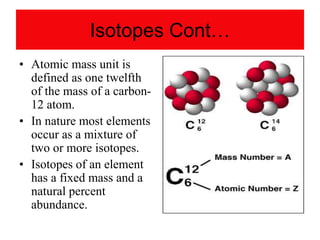
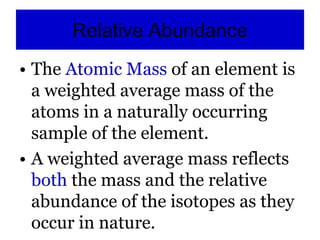
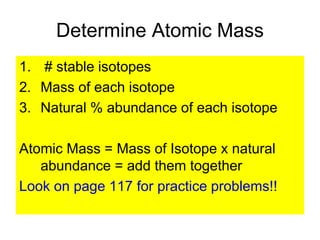
3) It defines important atomic properties including atomic number, mass number, isotopes, and how to calculate atomic mass.
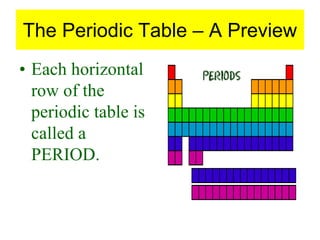
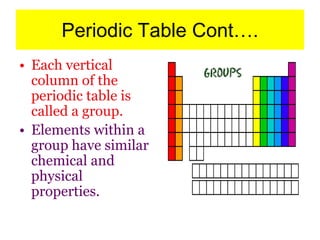
4) It provides an overview of how the periodic table organizes elements based on these atomic properties.