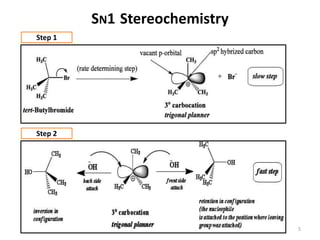
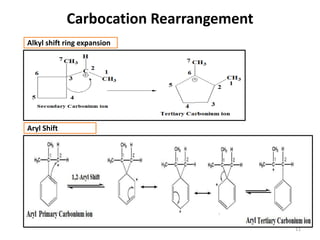
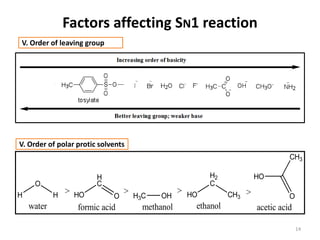
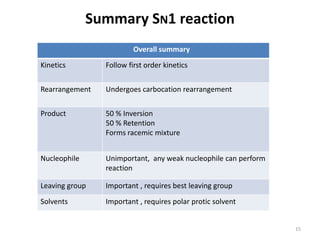
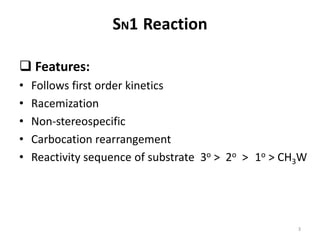
SN1 reactions follow first-order kinetics. They proceed through a carbocation intermediate and can undergo carbocation rearrangement. The reaction is non-stereospecific, resulting in a racemic mixture of products with 50% inversion and 50% retention of configuration. Carbocation stability is influenced by inductive, hyperconjugation, and resonance effects. The reactivity of the substrate depends on the stability of the carbocation and follows the order of tertiary > secondary > primary > methyl substrates. The reaction requires a good leaving group and is favored in polar protic solvents.

![SN1 Reaction Mechanism
4
Example
Rate= k[RBr] follows first order kinetics
Mechanism](https://image.slidesharecdn.com/demolectureoc-210220161640/85/SN1-Reaction-4-320.jpg)