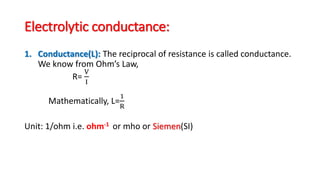
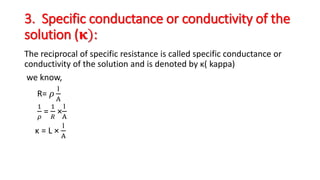
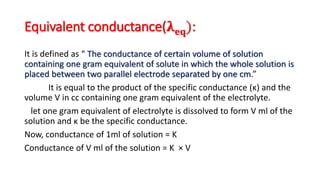
This document discusses key concepts in electrochemistry including:
- Electrochemistry deals with chemical and physical processes involving the production or consumption of electricity.
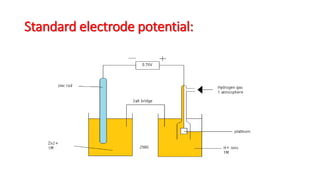
- Electrode potential is the potential difference that exists between a metal and its ions in solution, arising from their relative tendencies to undergo oxidation or reduction reactions.
- Standard hydrogen electrode is used as a reference electrode to measure standard electrode potentials of other half-cells.
- Standard electrode potential of a half-cell indicates its voltage when connected to the standard hydrogen electrode under standard conditions.
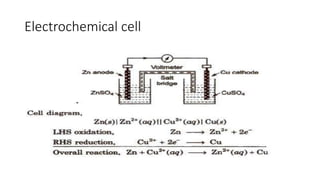
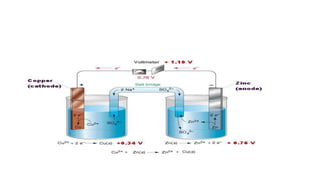
- Electromotive force is the difference in potential between the cathode and anode half-cells of an electrochemical cell.