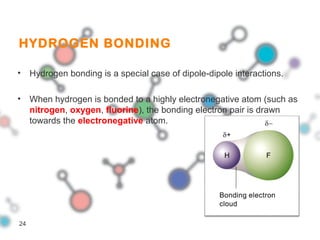
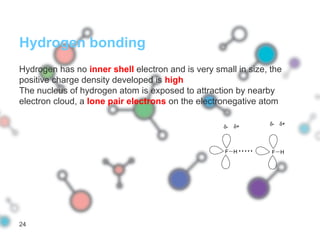
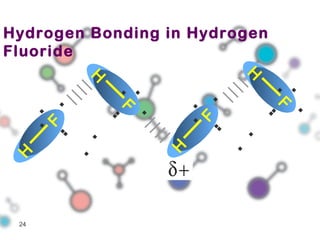
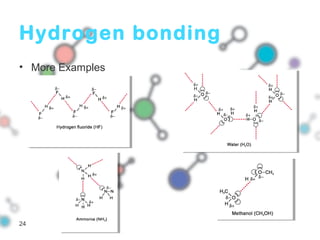
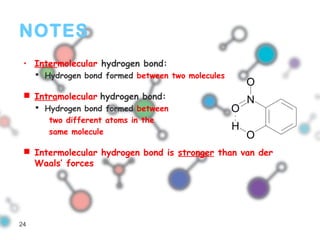
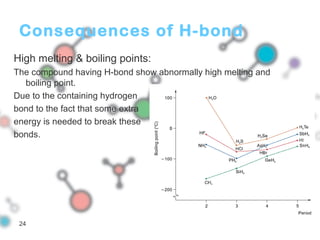
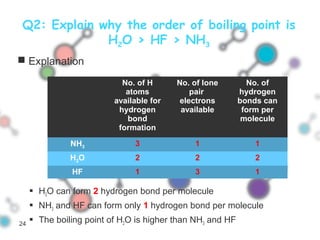
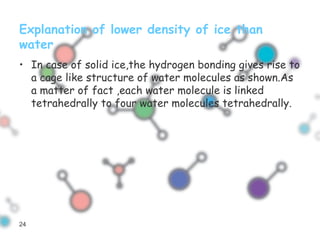
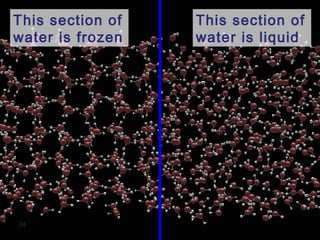
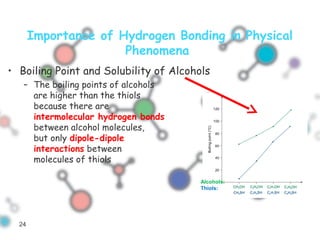
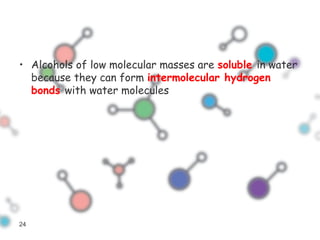
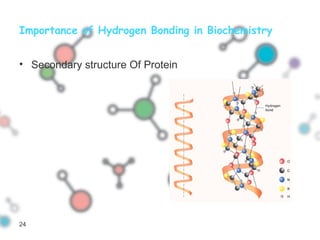
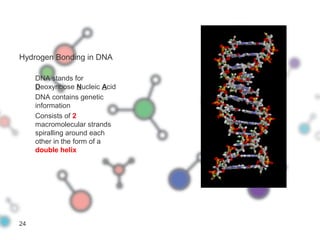
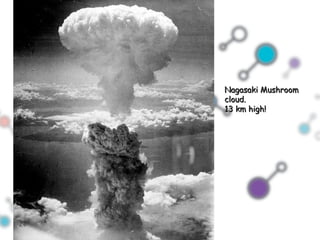
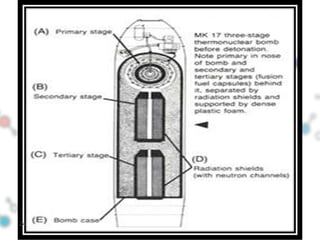
Hydrogen bonding occurs when a hydrogen atom bonded to a highly electronegative atom such as nitrogen, oxygen or fluorine interacts with another electronegative atom. This leads to compounds having higher boiling points than expected due to the need to overcome hydrogen bonding interactions. Hydrogen bonding plays important roles in determining the structure of materials like DNA and proteins and was also utilized in the hydrogen bomb to dramatically increase its explosive yield.