Alkanes
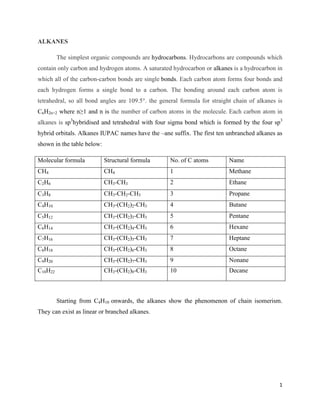
- 1. 1 ALKANES The simplest organic compounds are hydrocarbons. Hydrocarbons are compounds which contain only carbon and hydrogen atoms. A saturated hydrocarbon or alkanes is a hydrocarbon in which all of the carbon-carbon bonds are single bonds. Each carbon atom forms four bonds and each hydrogen forms a single bond to a carbon. The bonding around each carbon atom is tetrahedral, so all bond angles are 109.5°. the general formula for straight chain of alkanes is CnH2n+2 where n≥1 and n is the number of carbon atoms in the molecule. Each carbon atom in alkanes is sp3 hybridised and tetrahedral with four sigma bond which is formed by the four sp3 hybrid orbitals. Alkanes IUPAC names have the –ane suffix. The first ten unbranched alkanes as shown in the table below: Molecular formula Structural formula No. of C atoms Name CH4 CH4 1 Methane C2H6 CH3-CH3 2 Ethane C3H8 CH3-CH2-CH3 3 Propane C4H10 CH3-(CH2)2-CH3 4 Butane C5H12 CH3-(CH2)3-CH3 5 Pentane C6H14 CH3-(CH2)4-CH3 6 Hexane C7H16 CH3-(CH2)5-CH3 7 Heptane C8H18 CH3-(CH2)6-CH3 8 Octane C9H20 CH3-(CH2)7-CH3 9 Nonane C10H22 CH3-(CH2)8-CH3 10 Decane Starting from C4H10 onwards, the alkanes show the phenomenon of chain isomerism. They can exist as linear or branched alkanes.
- 2. 2 IUPAC SYSTEM OF CHEMICAL NOMENCLATURE The system of nomenclature used to name organic compounds was developed by the International Union of Pure and Applied Chemistry (IUPAC). A root identifies the longest continuous chain of carbon atoms. A suffix identifies the main functional group in the molecule. A set of prefixes identifies the numbers andpositions of the substituents (groups which are attached to the longest chain). (Alkyl groups are substituents which contain a carbon chain.) IUPAC NOMENCLATURE OF ALKANES Branched which is the chain alkanes are named according to the following rules: Step 1 Choose the longest continuous chain of carbon atoms and this chain determines the parent name for alkanes. Step 2 Number the longest chain beginning with the end of the chain nearer the substituent. Step 3 Use rule number 2 to locate the position of the substituent where the position and the name of the substituent must be written in front of the parent chain. Step 4 If two or more substituent are present, give each substituent a number corresponding to its location on the longest chain. The substituent should be listed alphabetically. In alphabetizing, the prefixes di, tri, tetra, sec- and tert- are ignored except iso and neo. Step 5 If two substituents are present on the same carbon atoms, use that number twice.
- 3. 3 Step 6 If two or more identical substituent are present, use prefixes di-(two identical substituent), tri-(three identical substituent), tetra-(four identical substituent). Commas are used to separate numbers from each other. Step 7 If there are two chains of equal length as the parent chain, choose the chain with the greaternumber of substituent. Step 8 If branching occurs at an equal distance from either and of the longest chain, choose the name that gives the lower number at the first point of different.
- 4. 4 CYCLOALKANES Introduction Cycloalkanes which also called as naphthenes are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules. Cycloalkanes contain only carbon- carbon single bonds and are saturated hydrocarbons. The general formula of these compounds is CnH2n. It has less hydrogen atom than alkanes, because another carbon-carbon bond is needed to form the ring. Cycloalkanes are drawn in simple polygons in which the sides represent the carbon-carbon bonds. Know that each corner of polygon is a carbon atom attached to two hydrogen atoms. The single ring are named analogously to their normal alkanes counterpart of the same carbon count such as Cyclopropane, Cyclobutane, Cyclopentane, Cyclohexane, Cycloheptane, Cyclooctane, Cyclononane, Cyclodecane, Cycloundecane, Cyclododecane and etc. Examples of cycloalkanes The multiple rings in a molecule can share one or more common atoms such as spiro cyclic compounds, fused rings compounds and bridged ring compounds. One carbon atom shared between two rings known as spiro cyclic compounds. Fused ring compounds shared two common atoms and the bond between them. Last compound which shared two nonadjacent carbon atoms which are called bridged head carbon atoms is known as bridged ring compounds.
- 5. 5 Examples multiple ring of cycloalkanes Naming Cycloalkanes Saturated cyclic hydrocarbons are called cycloalkanes or alicyclic compounds. Cycloalkanes are named according to the IUPAC system by using the prefix cyclo-. Substituted cycloalkanes are named by rules similar to in open-chain alkanes. There are only two steps for most compounds: STEP 1: Find the parent. First of all count the number of carbon atoms in the ring and the number in the largest substituent. The compound is named as an alkyl-substituent if the number of carbon atoms in the ring is equal or greater than the number in the substituent. While when the number of carbon atom in the largest substituent is greater than the number in the ring, the compound is named as a cycloalkyl-substituted alkane. Exampels of alkyl-substituent cycloalkanes and cycloalkyl-substituent alkanes
- 6. 6 STEP 2: Number of substituent and write the name. For alkyl- or halo-substituent cycloalkanes, if there is one substituent, there is no need to show the number 1 of the substituent. If two or more substituents, choose a point of attachment as carbon 1 such that the second substituent has a low number as possible. If there still ambiguity number so that the third substituent has the low number as possible. If there still ambiguity, continue as before until a point of difference is found. For examples: NOT Right Answer Wrong Answer NOT NOT Right Answer Wrong Answer Wrong Answer
- 7. 7 a) When two or more different alkyl groups that could potentially receive the same number are present, number them by alphabetical priority, ignoring numerical prefixes such as di- and tri-. NOT Right Answer Wrong Answer b) If halogens are present, treat them just like alkyl groups. NOT Right Answer Wrong Answer
- 8. 8 REACTION OF ALKANES Alkanes are less reactive compared to alkenes, arenes and others.This is because carbon and hydrogen atoms in alkanes have similar electronegativity values making the C-H bond not polar. So, alkanes are not attacked by nucleophile and electrophile.Besides, alkanes do not have unpaired and unshared electron to be reactive towards acids or electrophiles.Alkanes undergo the following reactions: Combustion Complete combustion forms carbon dioxide and water whereas incomplete combustion forms carbon monoxide or carbon and water. The reaction is exothermic. Complete combustion of butane Incomplete combustion of butane Incomplete combustion causes pollution. Halogenation Reaction with halogen gas such as chlorine, bromine and iodine gas in the presence of ultraviolet light or high temperatures to form haloalkane or alkyl halide. The mechanism involved is free radical Mechanism of halogenation reaction i) Chain Initiation Step The Cl-Clcovelant bond undergoes hemolytic fission whereby the covalent bond breaks to form free radicals with aid of ultraviolet or high temperature. The process is endothermic and high energy from UV light or high temperature is used to break the covalent bond. Two chlorine free radicals,Cl are formed.
- 9. 9 ii) Chain Propagation Step The chlorine free radical is very reactive enough to remove hydrogen atom hydrogen atom from methane by breaking the C-H bond to form HCl and , metyl free radical. Methyl free radical ,then react with chlorine molecule to form chlorine free radical, Cl and Cl,chloromethane. The chlorine radical, Cl is reused in the above step and the process is repeated to produce more methyl free radical, and Cl, chlorine free radicals. iii) Chain Termination Step In this step, all the reactive free radicals will combine or react with one another to form neutral species. Naming of Haloalkanes Naming of haloalkanes also using the IUPAC nomenclature(International Union Pure and Applied Chemistry. There is several step can be use to naming the haloalkanes.First,identify the root name based on the longest chain containing halogen. Secondstep, theroot give the alkane
- 10. 10 part of the name.Third step,the of halogen defines the halogen prefixes such as chlorine the prefixes is chloro.Last but not list, the chain is numbered to give the halogen the lowest possible number.Then, the haloalkanes are named. The Example Naming of Haloalkane CH3CH2CH2Cl 1-chloropropane The functional group is identified as an alkane, therefore suffix is -ane and the longest continuous chain has three carbon therefore the root name is propane.After that, the substituent is chlorine, therefore prefix of chlorine is chloro.Last,the carbon requires numbering from the right as drawn, the substituent located at 1 on the carbon 1.Carbon first should be numbering on the near carbon to the substituent.The name of the haloalkane is 1-chloropropane.