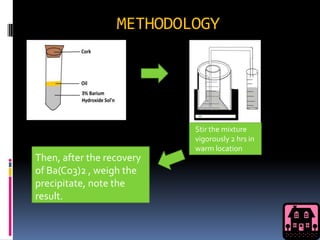
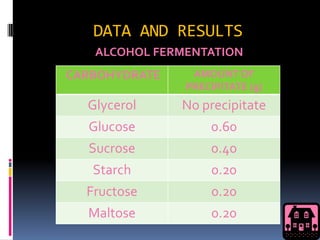
This document discusses alcohol fermentation by yeast. Yeast converts sugars like glucose, fructose, maltose and sucrose into ethanol and carbon dioxide through a process called alcoholic fermentation. Experiments showed that glucose produced the most barium carbonate precipitate, indicating it underwent the fastest fermentation. Other sugars like glycerol that can't be broken down into glucose did not produce precipitate.