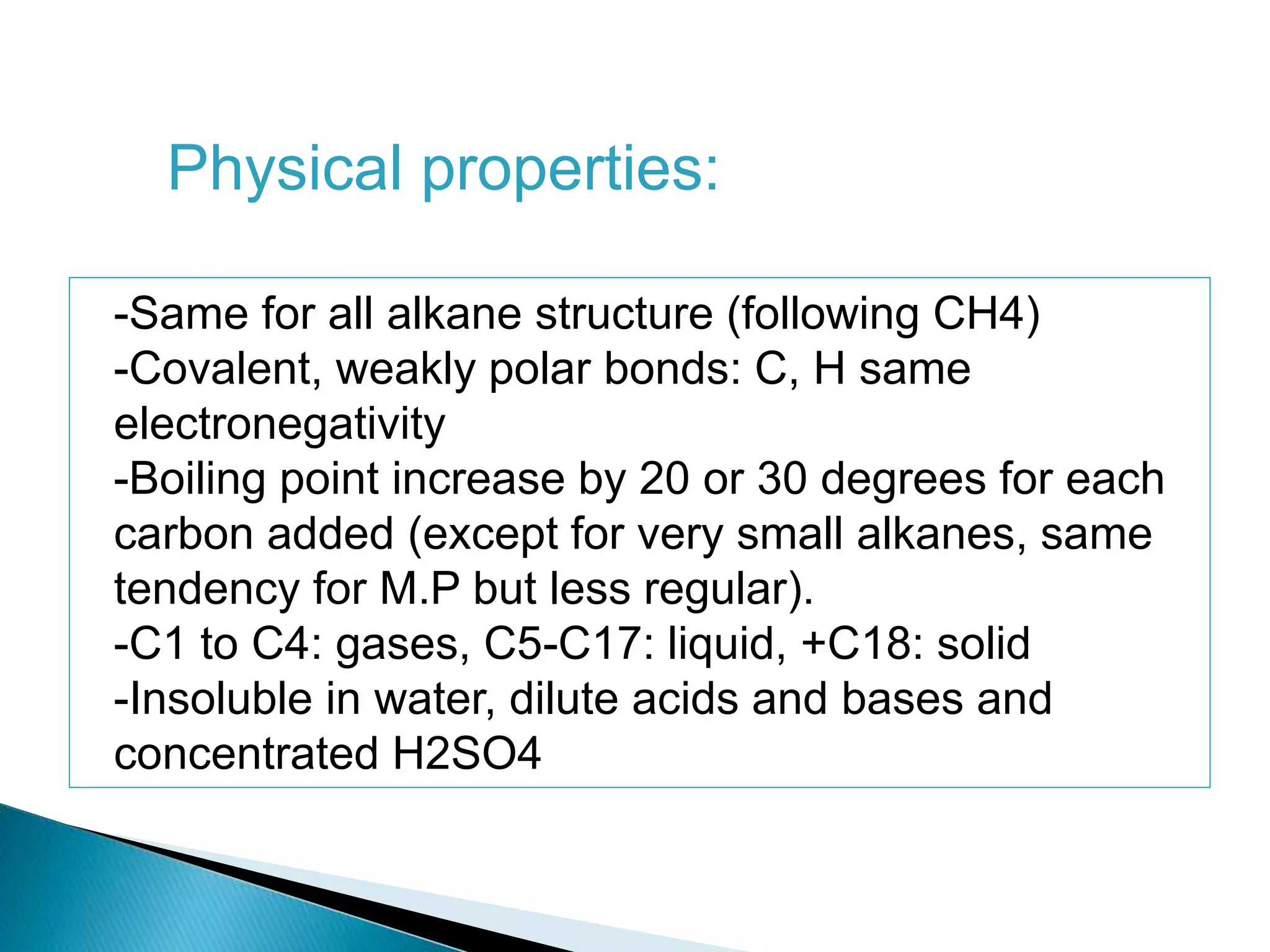
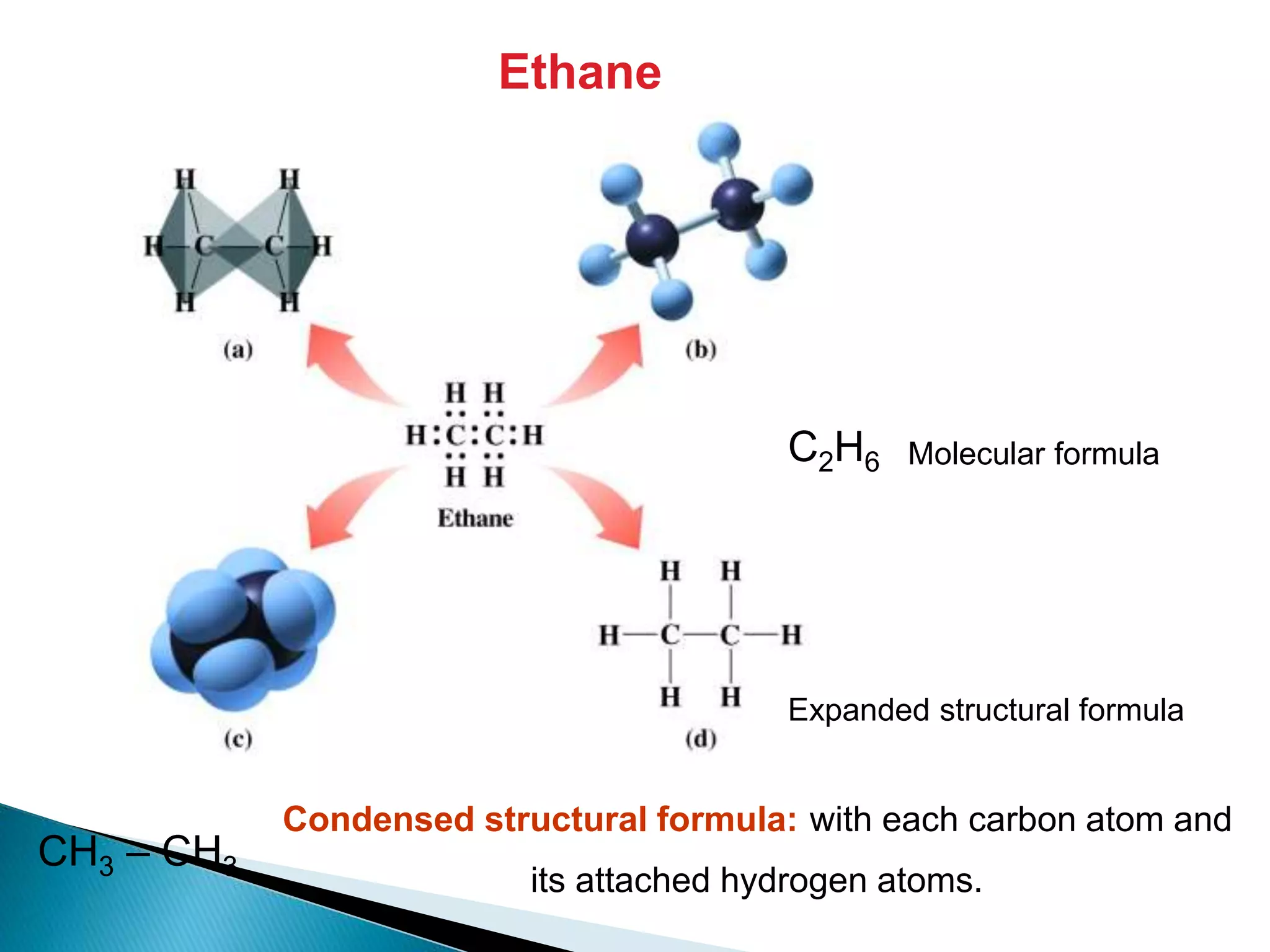
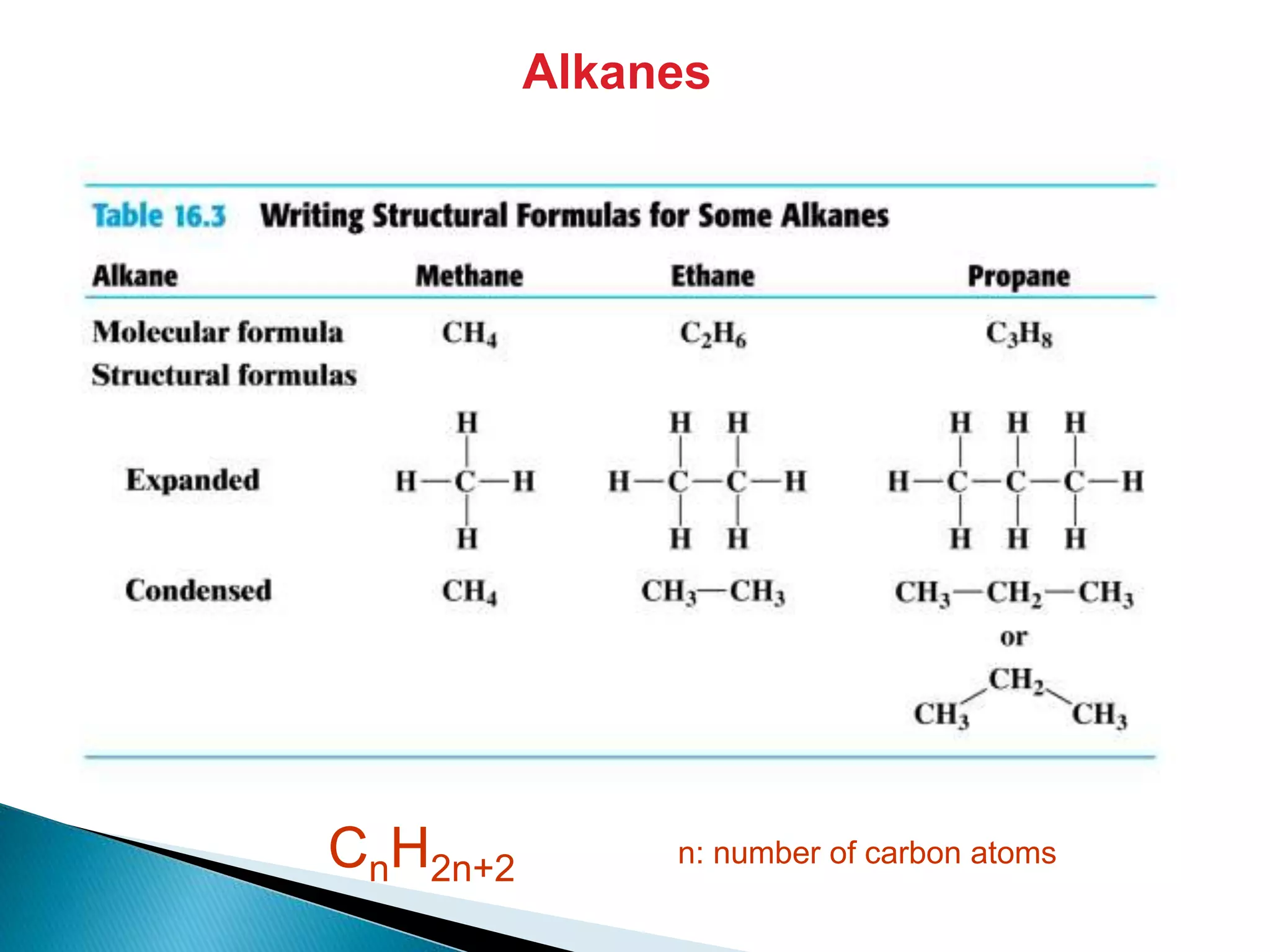
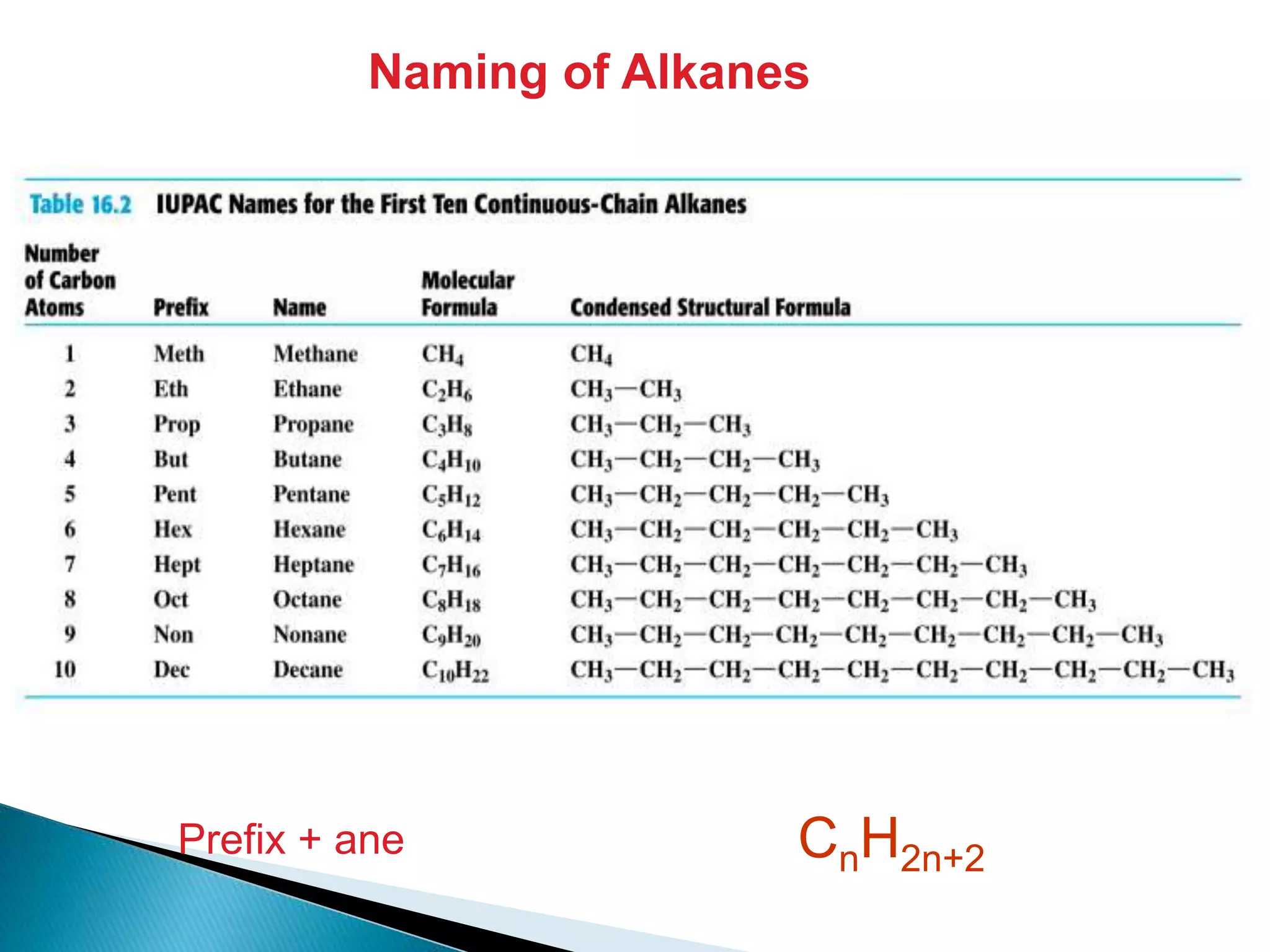
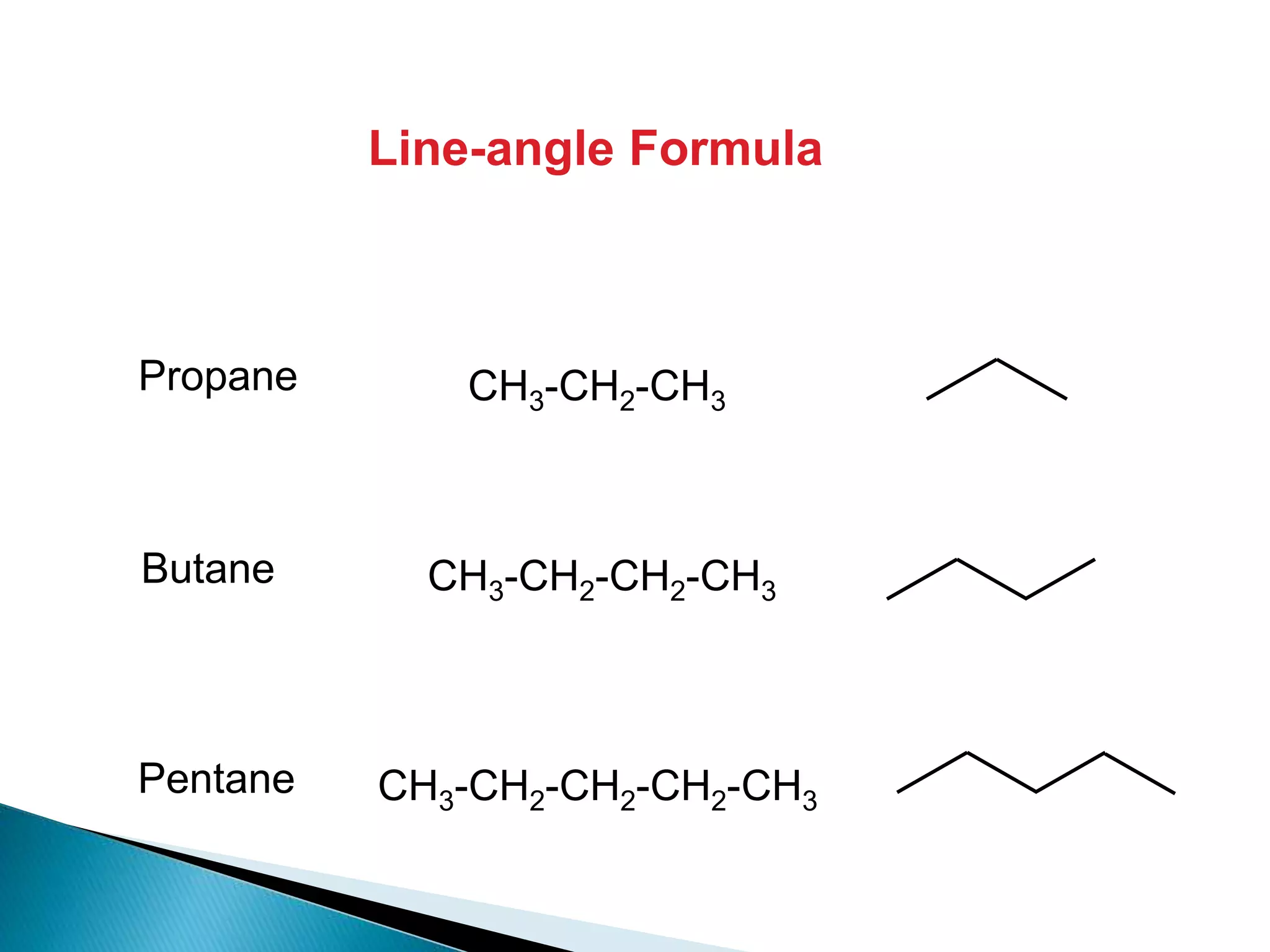
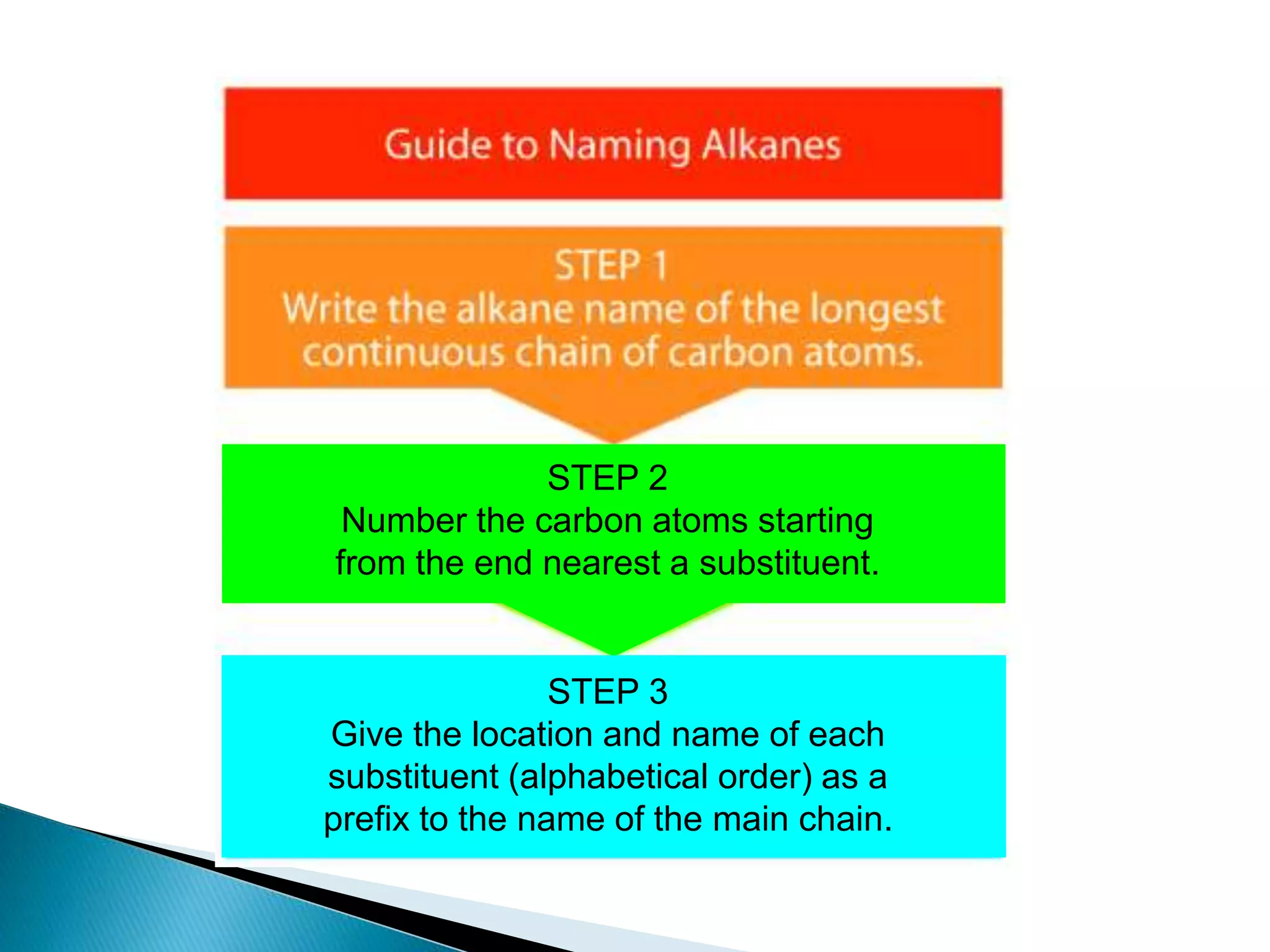
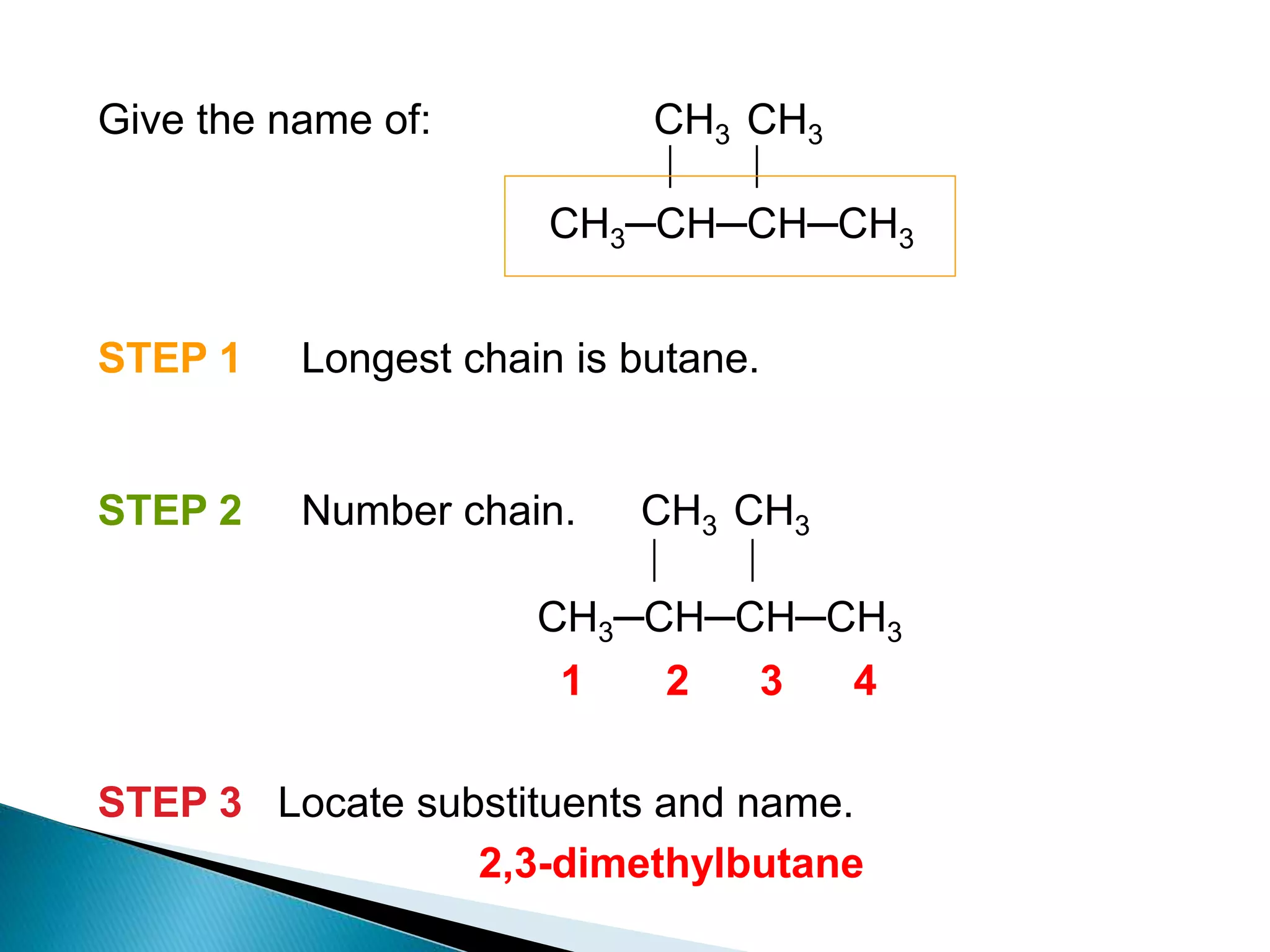
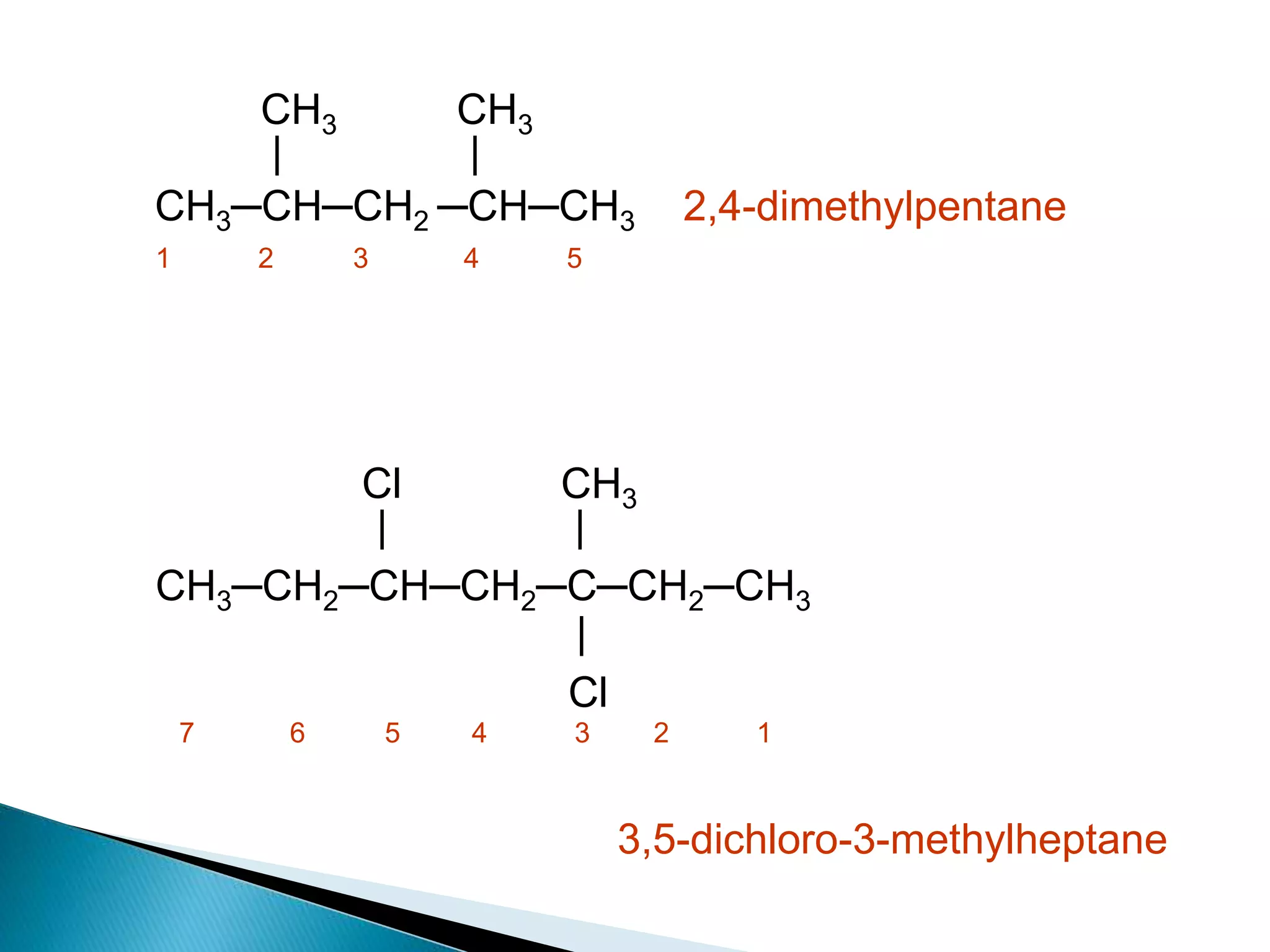
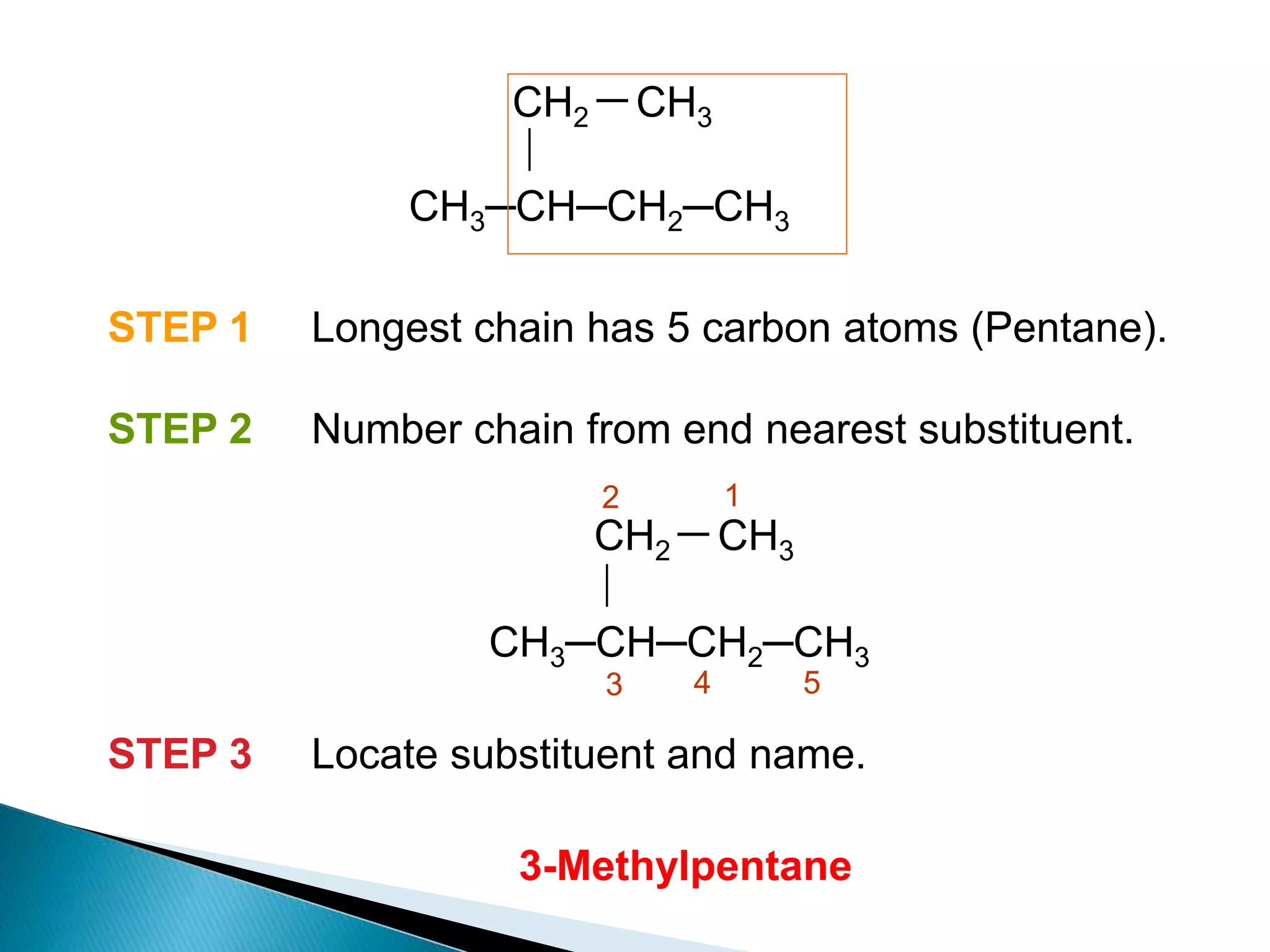
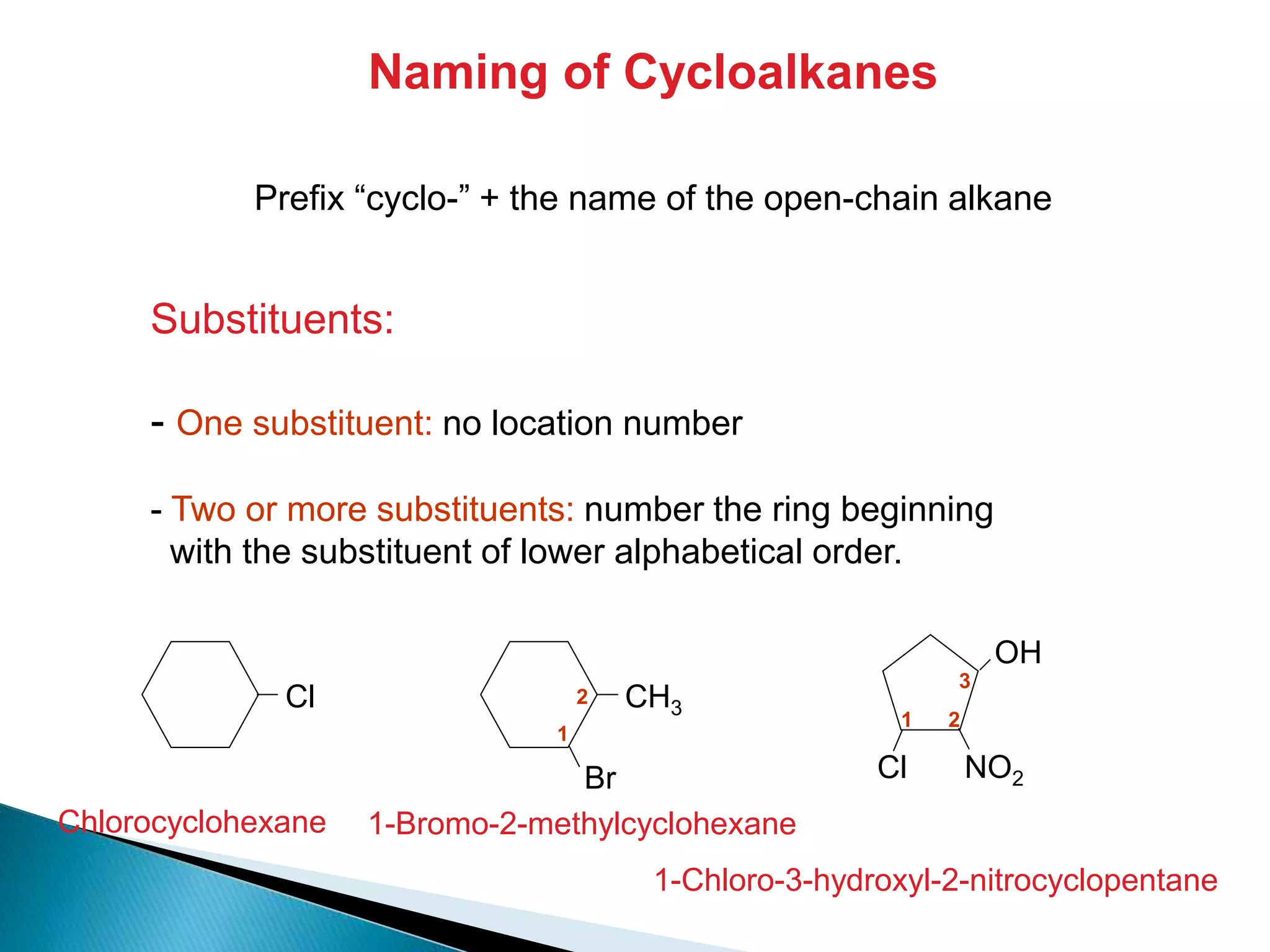
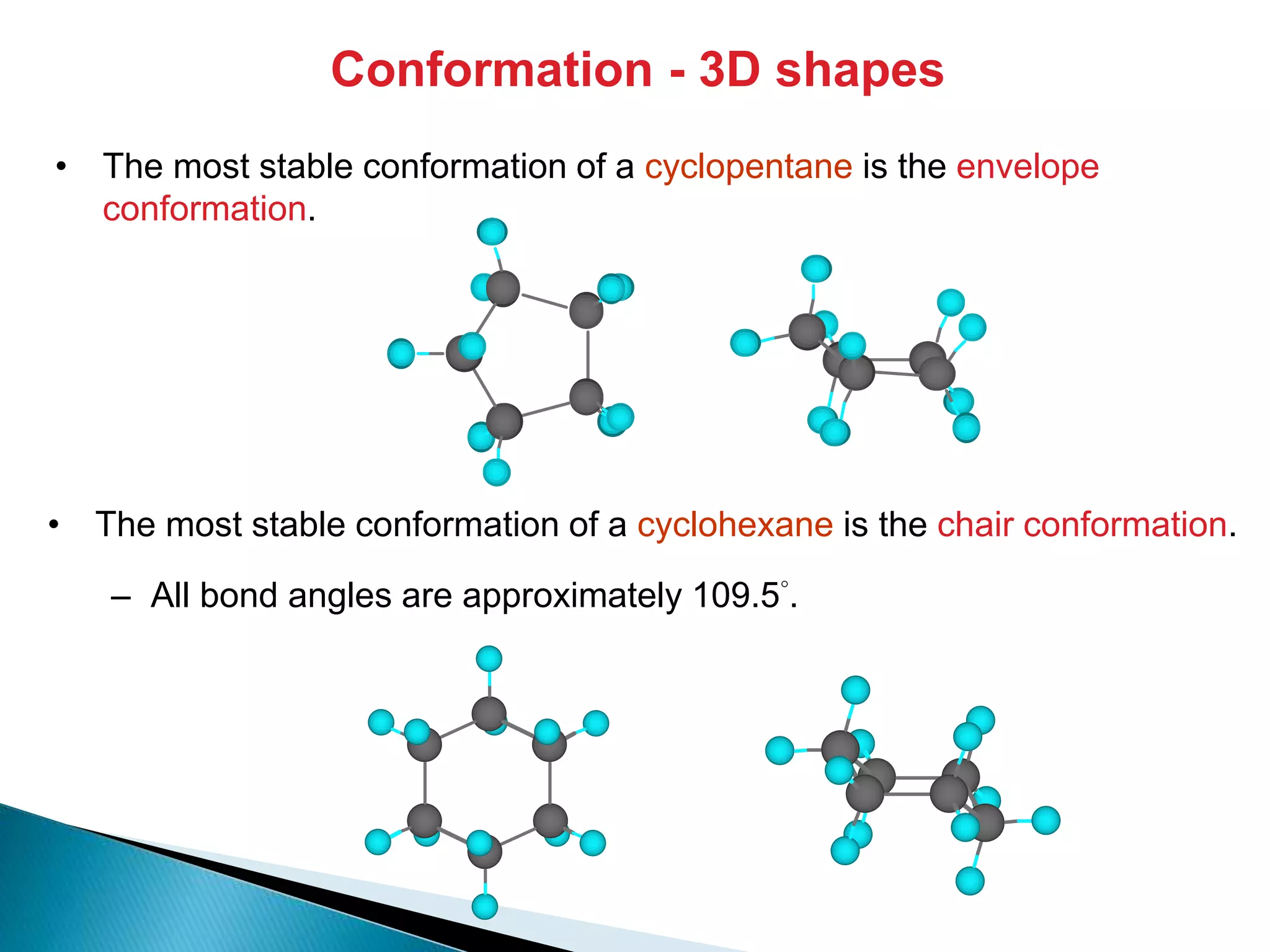
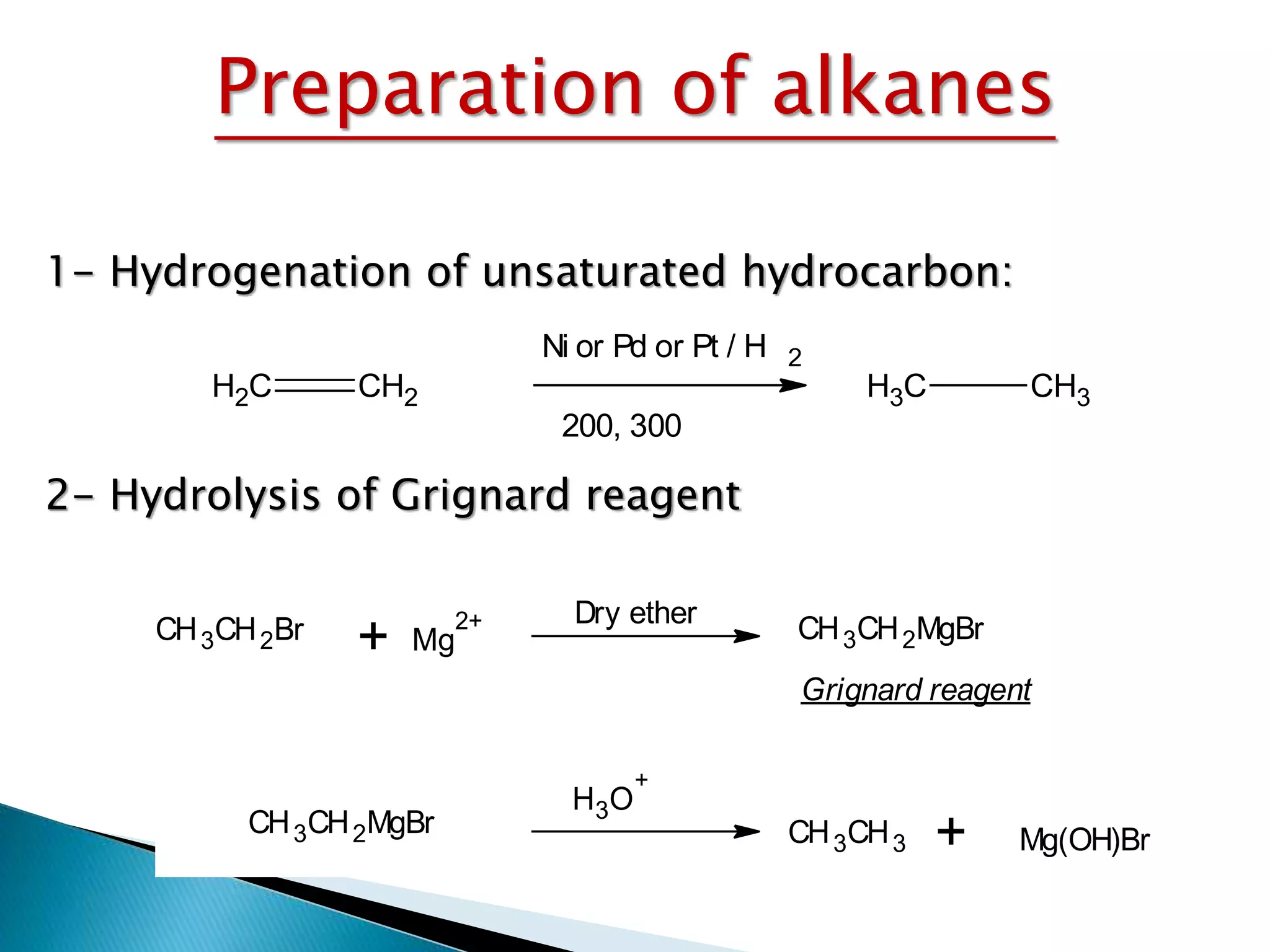
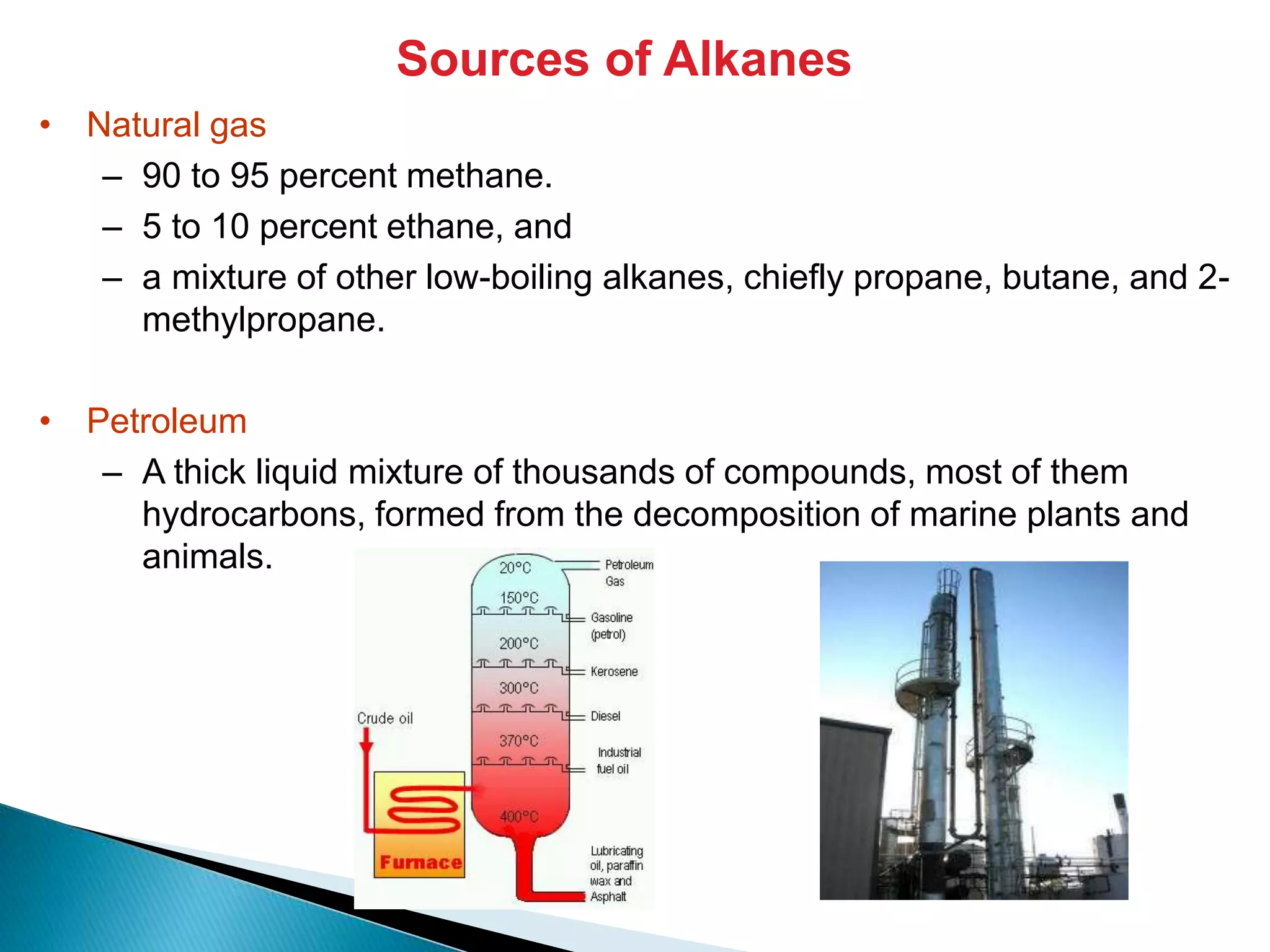
The document provides an overview of alkanes, including their molecular structure, properties, nomenclature, and reactions. Alkanes are a family of hydrocarbons with a general formula of CnH2n+2, characterized by single bonds and varying physical states based on carbon chain length. The document also discusses the preparation and chemical reactions of alkanes, highlighting their low reactivity and sources such as natural gas and petroleum.