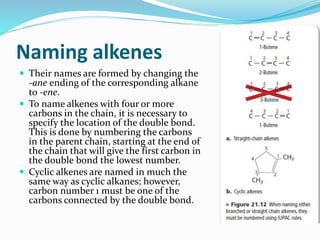
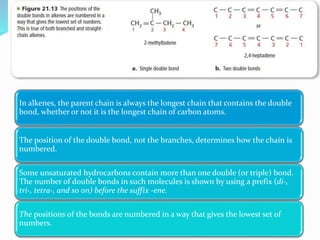
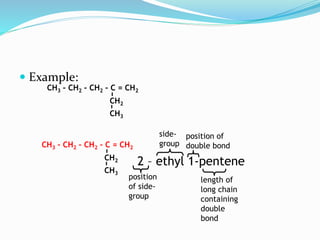
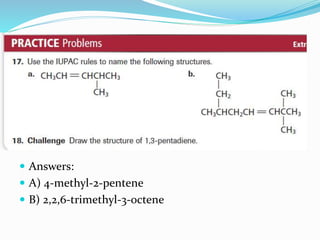
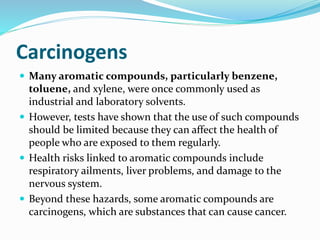
This document provides an introduction to organic chemistry, including definitions of key terms and concepts. It discusses:
- The early history of organic chemistry and the discovery that organic compounds could be synthesized in the lab.
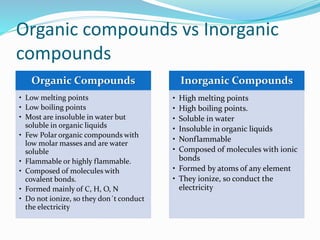
- The main differences between organic and inorganic compounds in terms of their properties and bonding.
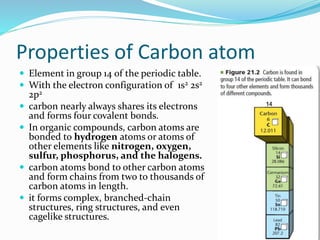
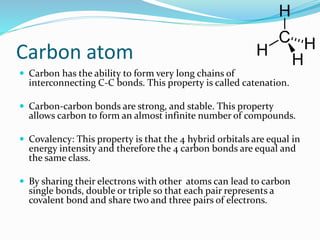
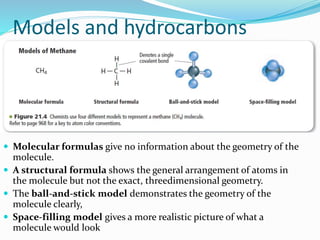
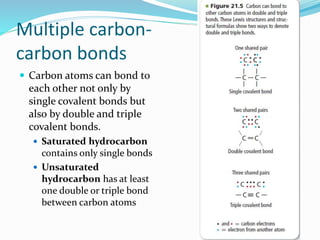
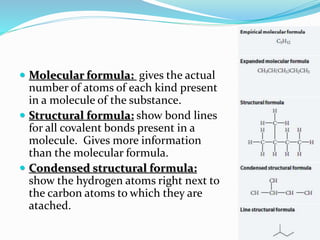
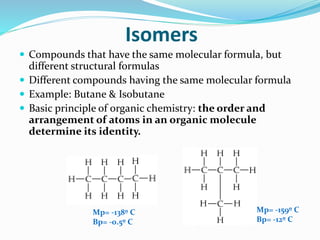
- The central role of carbon atoms in organic compounds and their ability to form chains and complex structures through catenation.
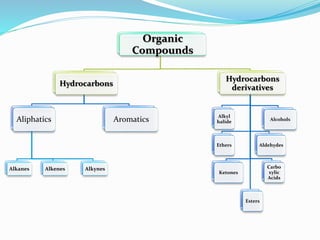
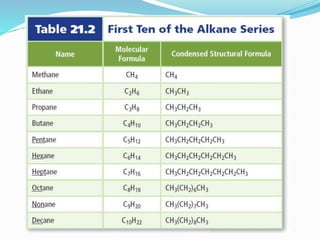
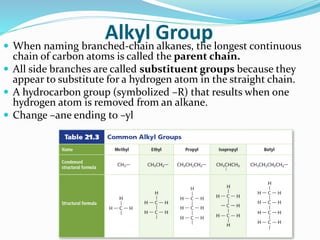
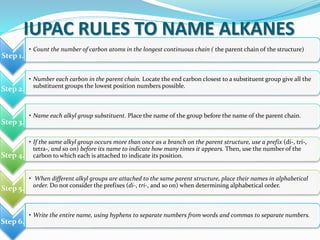
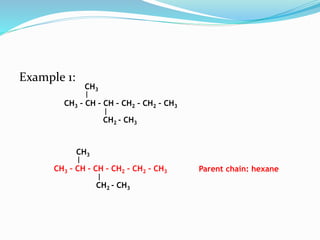
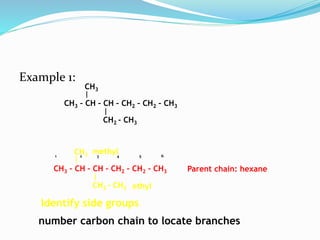
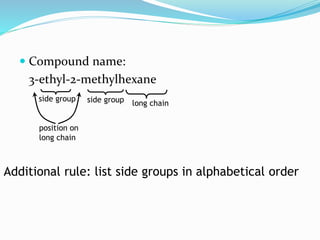
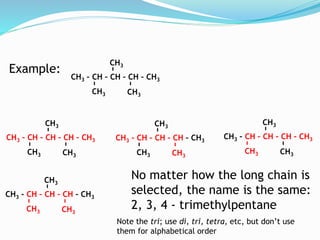
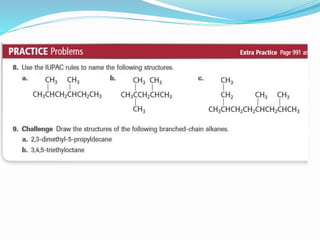
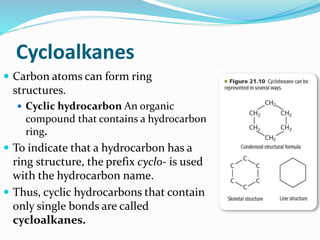
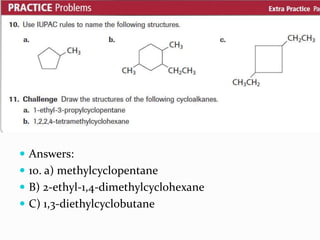
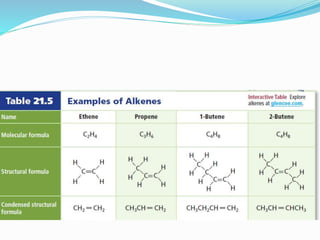
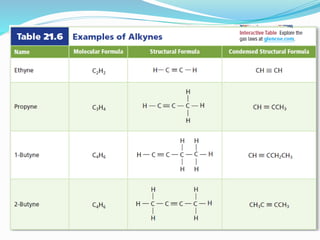
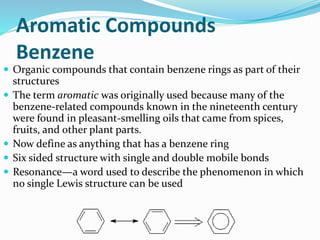
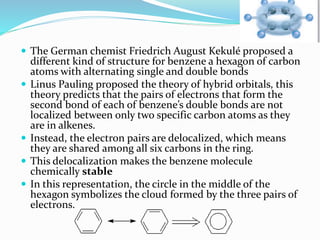
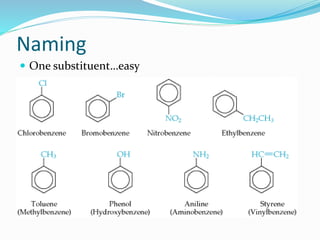
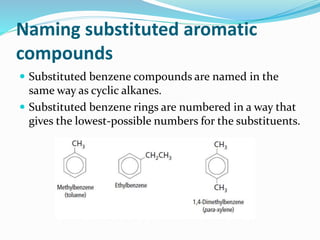
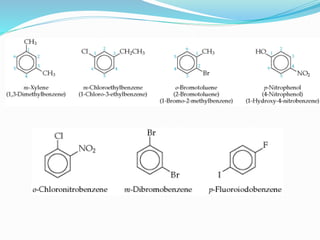
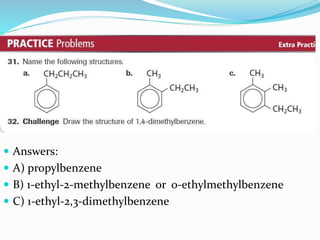
- The different classes of hydrocarbons including alkanes, alkenes, alkynes, aromatics, and their IUPAC naming conventions.
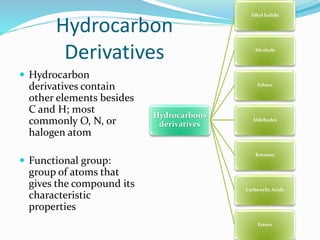
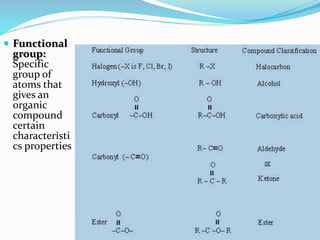
- Important organic functional groups derived from hydrocarbons like alkyl halides, alcohols, ethers, al