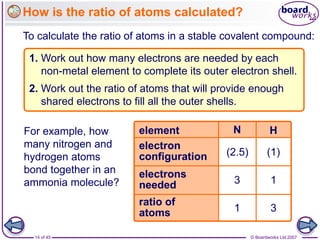
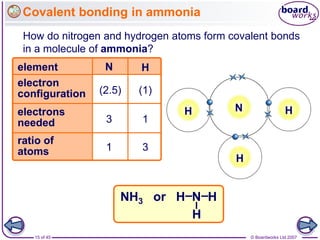
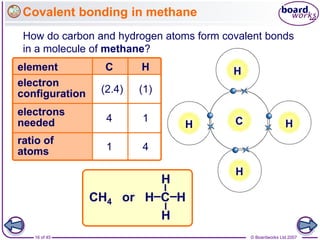
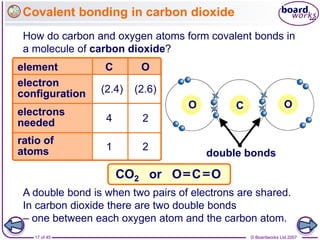
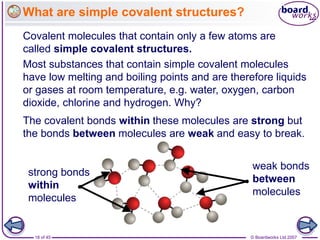
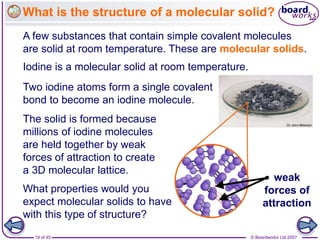
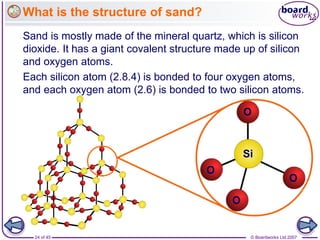
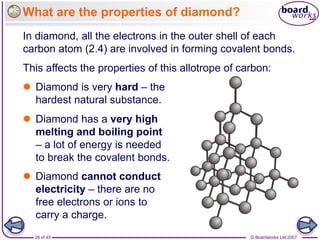
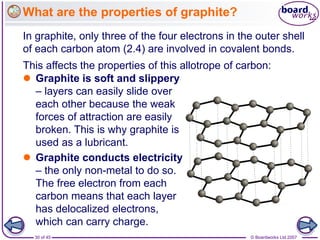
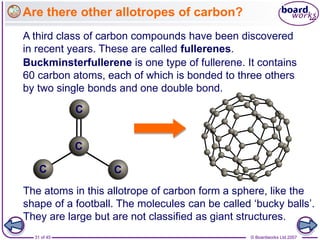
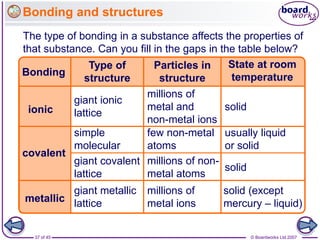
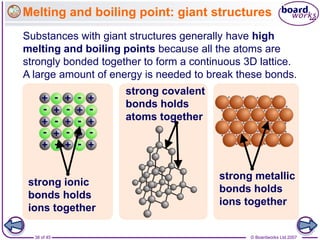
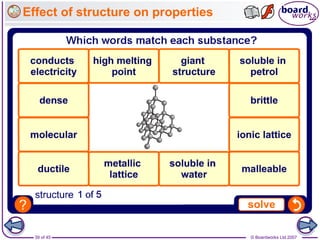
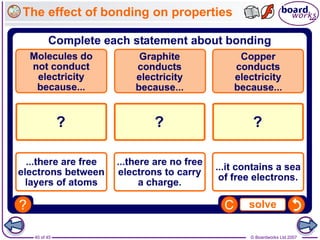
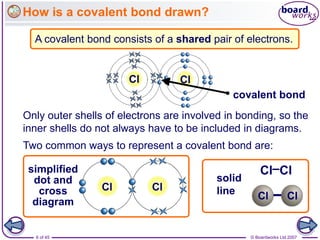
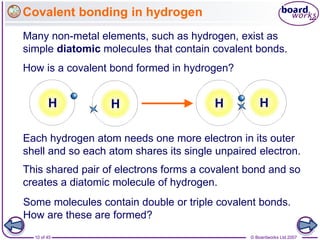
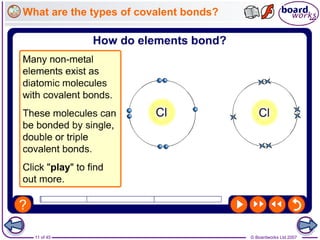
Atoms form bonds to achieve stable electron configurations. Covalent bonds form when atoms share valence electrons to fill their outer shells. Different bonding structures lead to varied properties. Diamond has a giant covalent structure where each carbon atom bonds to four others in a 3D network, giving it properties like hardness. Graphite also contains carbon but its layers can slide due to weaker bonds between layers, making it soft.
![© Boardworks Ltd 2007
13 of 45
Covalent bonding in water
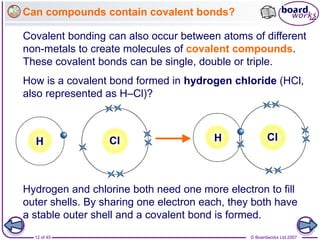
Compounds can contain more than one covalent bond.
The oxygen atom shares 1
electron with 1 hydrogen
atom, and a second
electron with another
hydrogen atom.
H H
O
Oxygen (2.6) needs 2 more electrons, but hydrogen [1] only
needs 1 more. How can these three elements be joined by
covalent bonding?
What is the name of the molecule that is formed?
H2O (or H–O–H) is water.](https://image.slidesharecdn.com/26-210213040656/85/26-covalent-bonding-13-320.jpg)