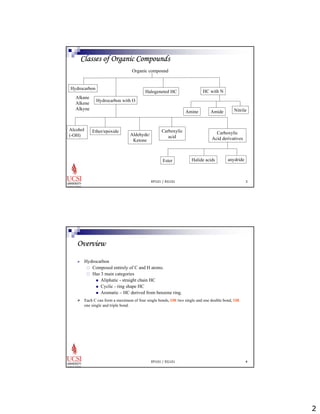
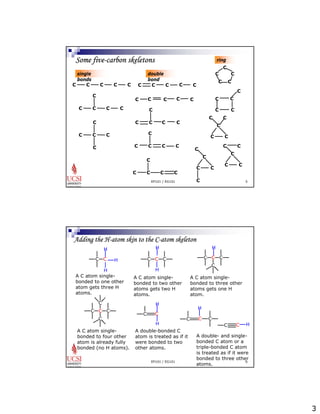
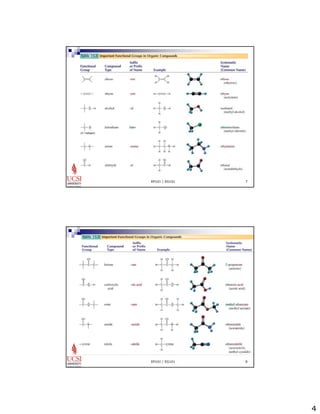
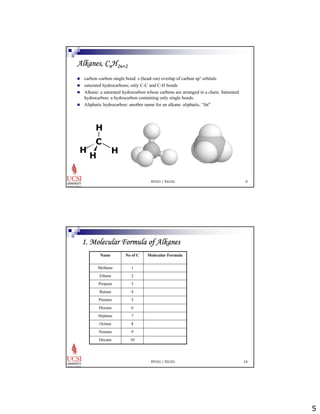
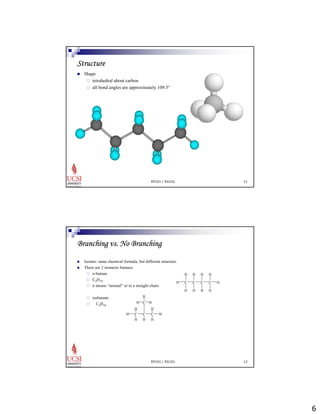
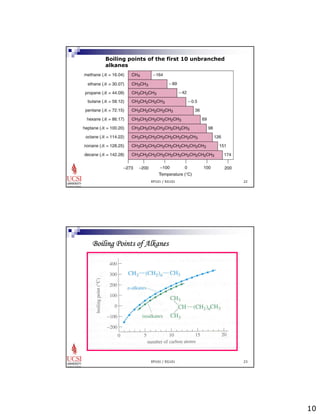
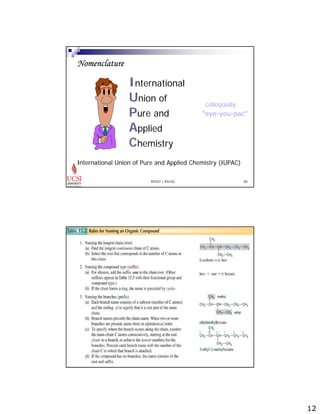
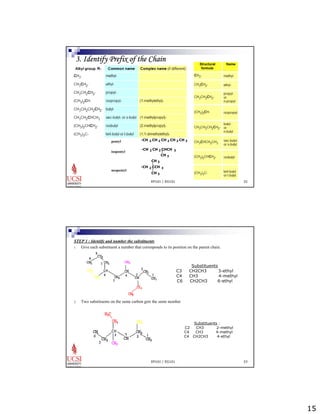
The document provides information about aliphatic hydrocarbons, specifically alkanes. It begins with learning outcomes which state that students will understand organic compound families, explain alkane structure and properties, name organic compounds using IUPAC rules, describe alkane isomers and synthesis reactions. The document then covers classes of organic compounds including hydrocarbons, alkane properties such as physical states and solubility, IUPAC nomenclature rules, cyclic alkane naming, and alkane reaction and synthesis methods like hydrogenation of alkenes.