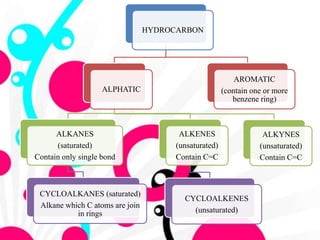
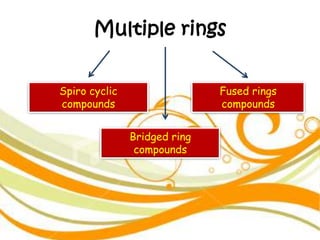
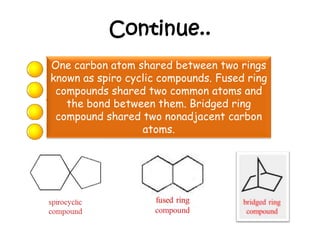
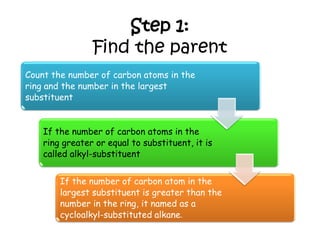
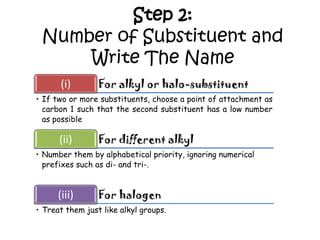
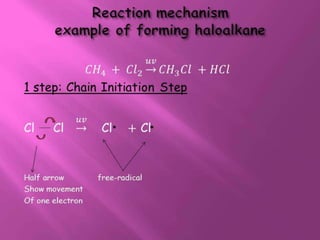
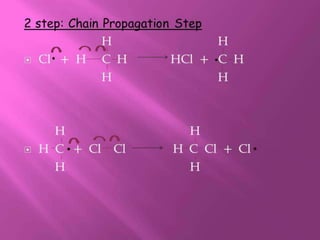
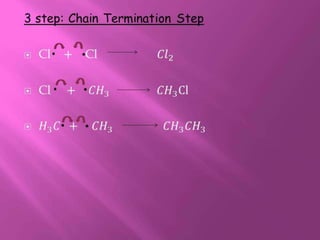
1. The document discusses the basics of organic chemistry, focusing on hydrocarbons and specifically alkanes.
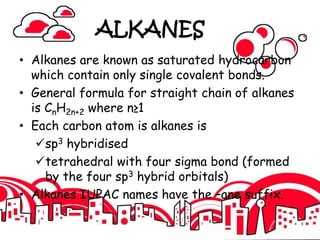
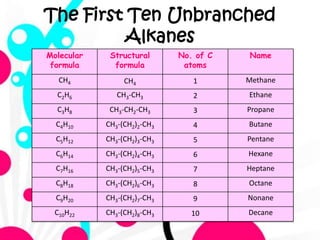
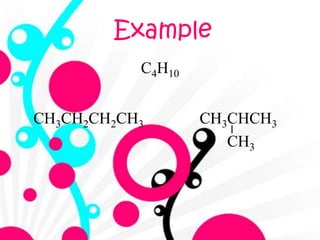
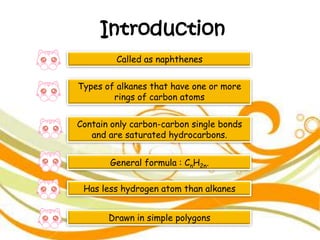
2. It defines alkanes as saturated hydrocarbons containing only single bonds between carbon atoms. The general formula for alkanes is CnH2n+2.
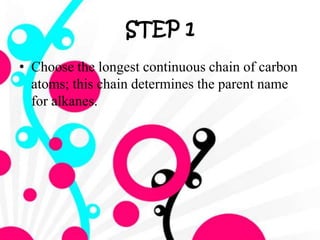
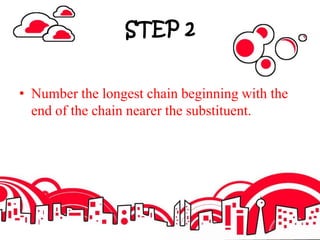
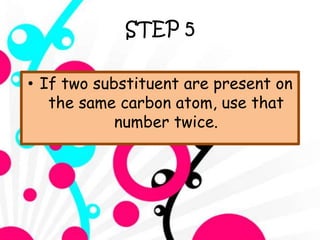
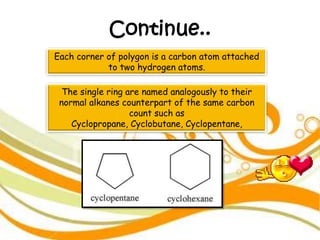
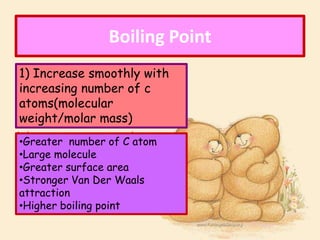
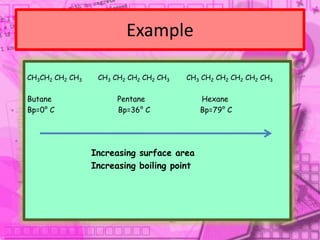
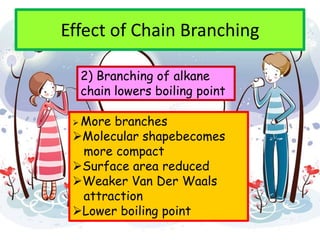
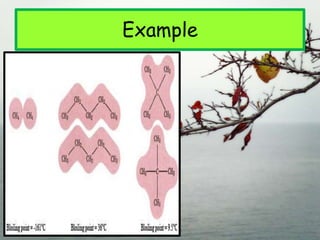
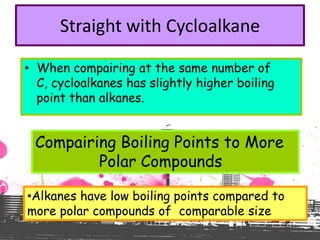
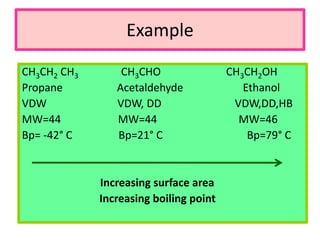
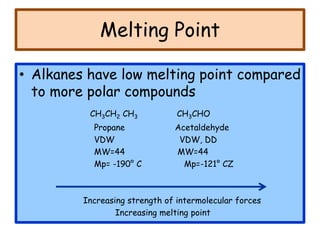
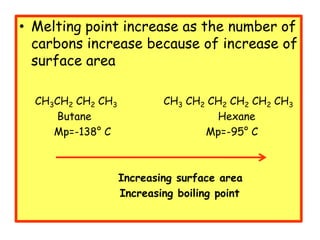
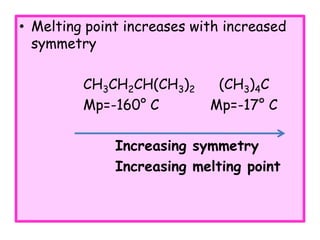
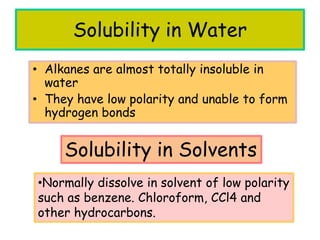
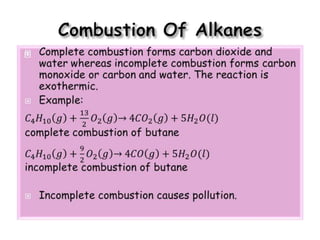
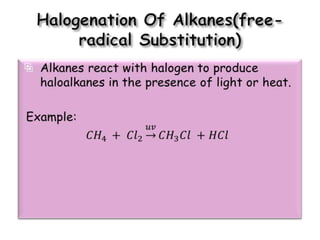
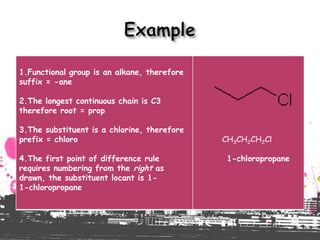
3. The document outlines IUPAC nomenclature rules for naming branched alkanes and cycloalkanes. Physical properties like boiling point, melting point, and solubility are also addressed.