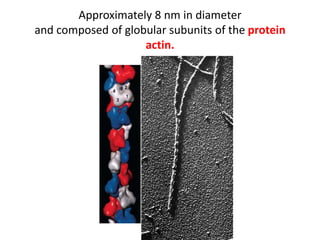
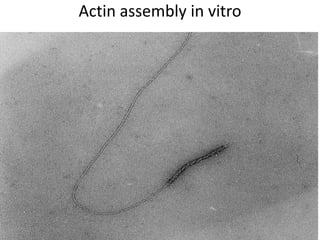
1) Actin microfilaments are composed of globular actin subunits that polymerize to form filaments approximately 8 nm in diameter.
2) Actin polymerization is regulated by actin-binding proteins that nucleate, depolymerize, sever, cross-link, or cap actin filaments.
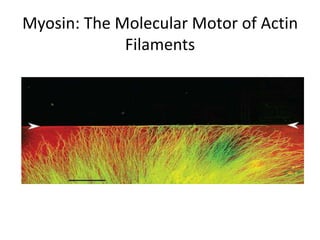
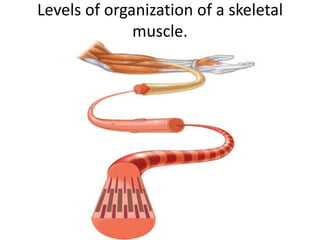
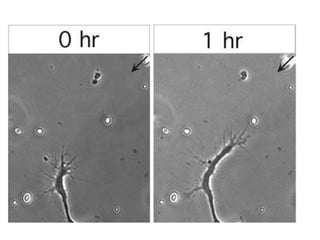
3) In nonmuscle and muscle cells, actin microfilaments interact with myosin to generate forces responsible for cell motility, shape changes, and muscle contraction.