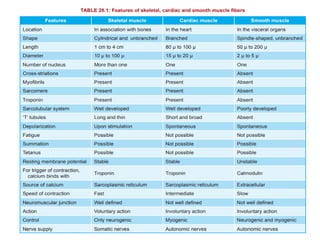
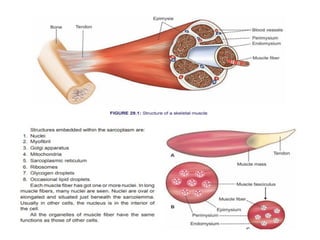
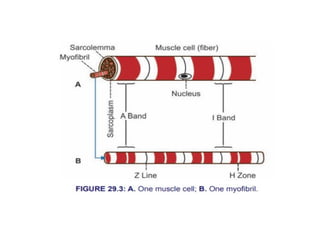
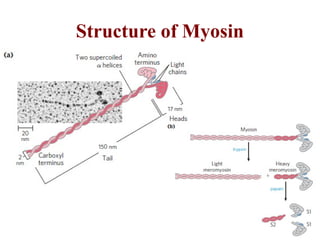
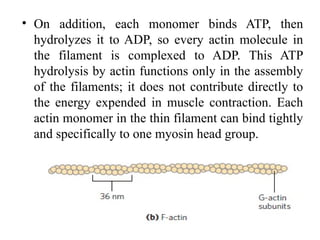
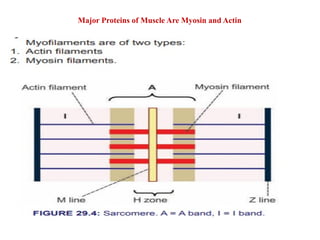
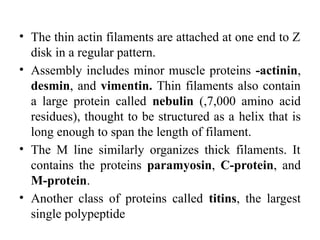
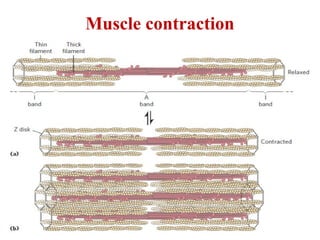
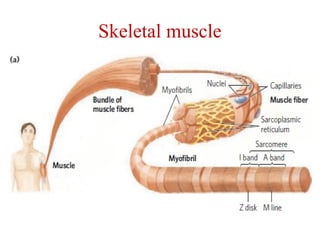
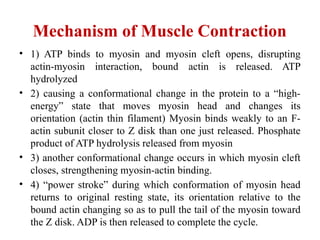
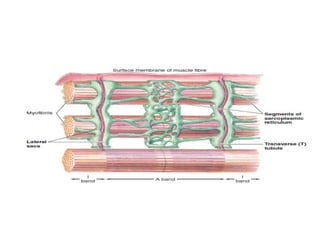
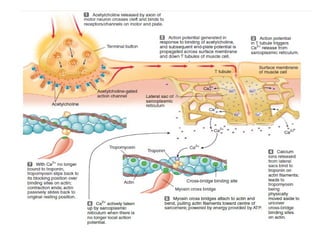
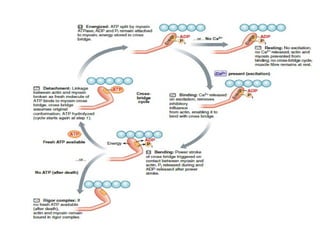
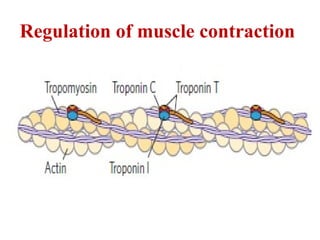
The document outlines the structure and function of muscle proteins myosin and actin, detailing myosin's composition and its role in muscle contraction through the sliding filament mechanism. It explains how actin filaments interact with myosin heads during contraction, regulated by tropomyosin and troponin in response to calcium ions released from the sarcoplasmic reticulum. This interaction is essential for muscle fibers to contract properly, with the document also referencing sources for further reading.