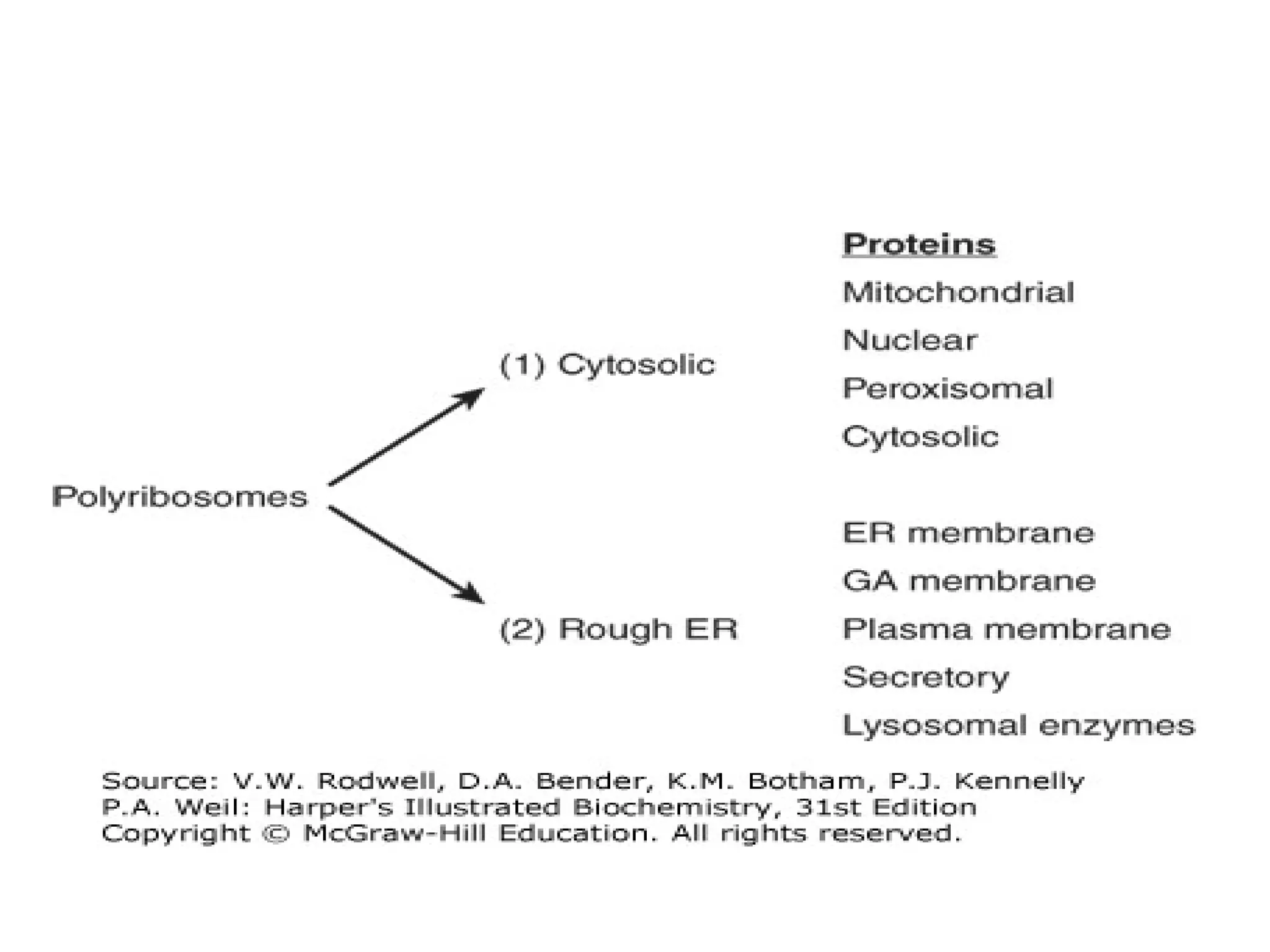
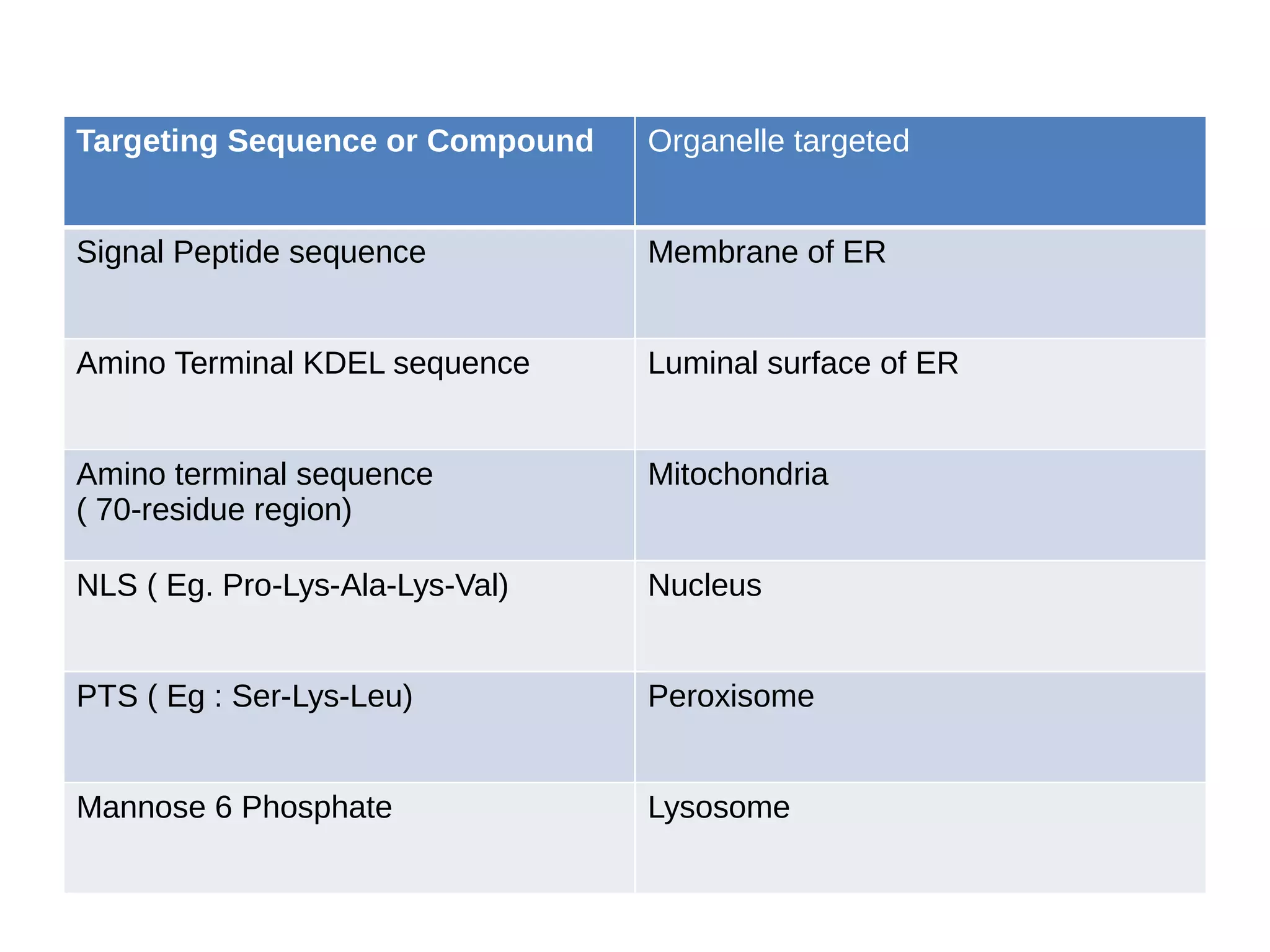
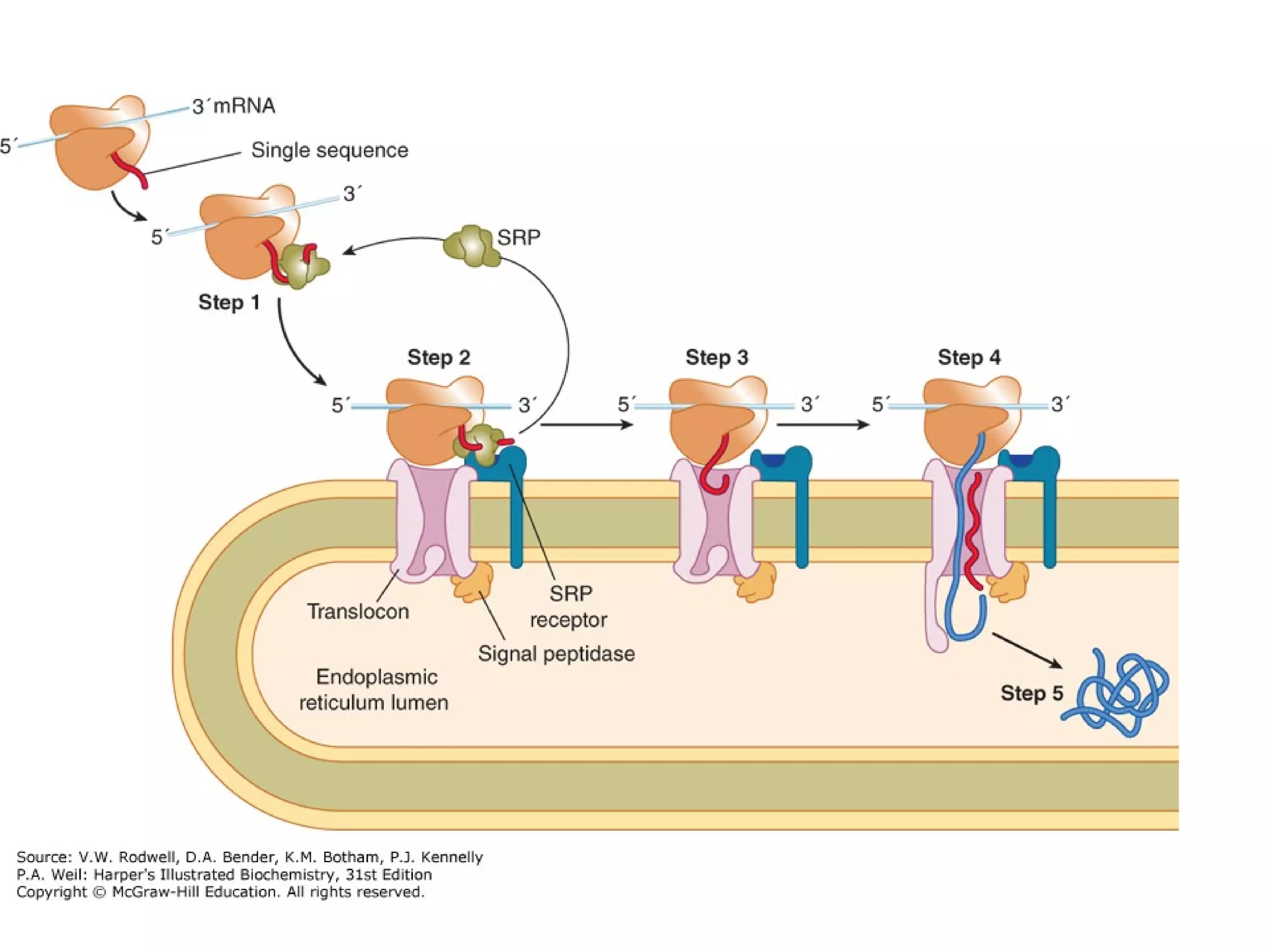
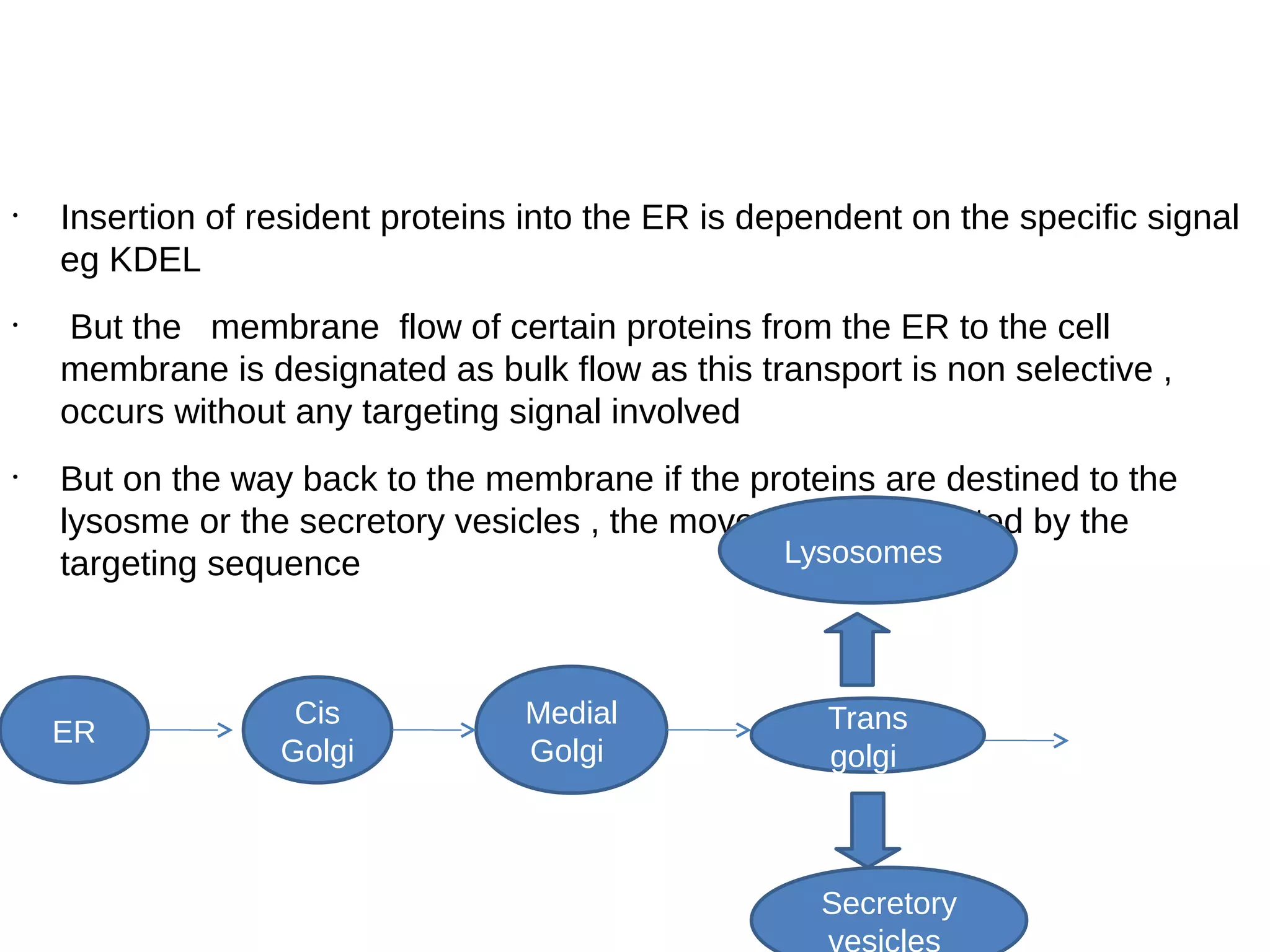
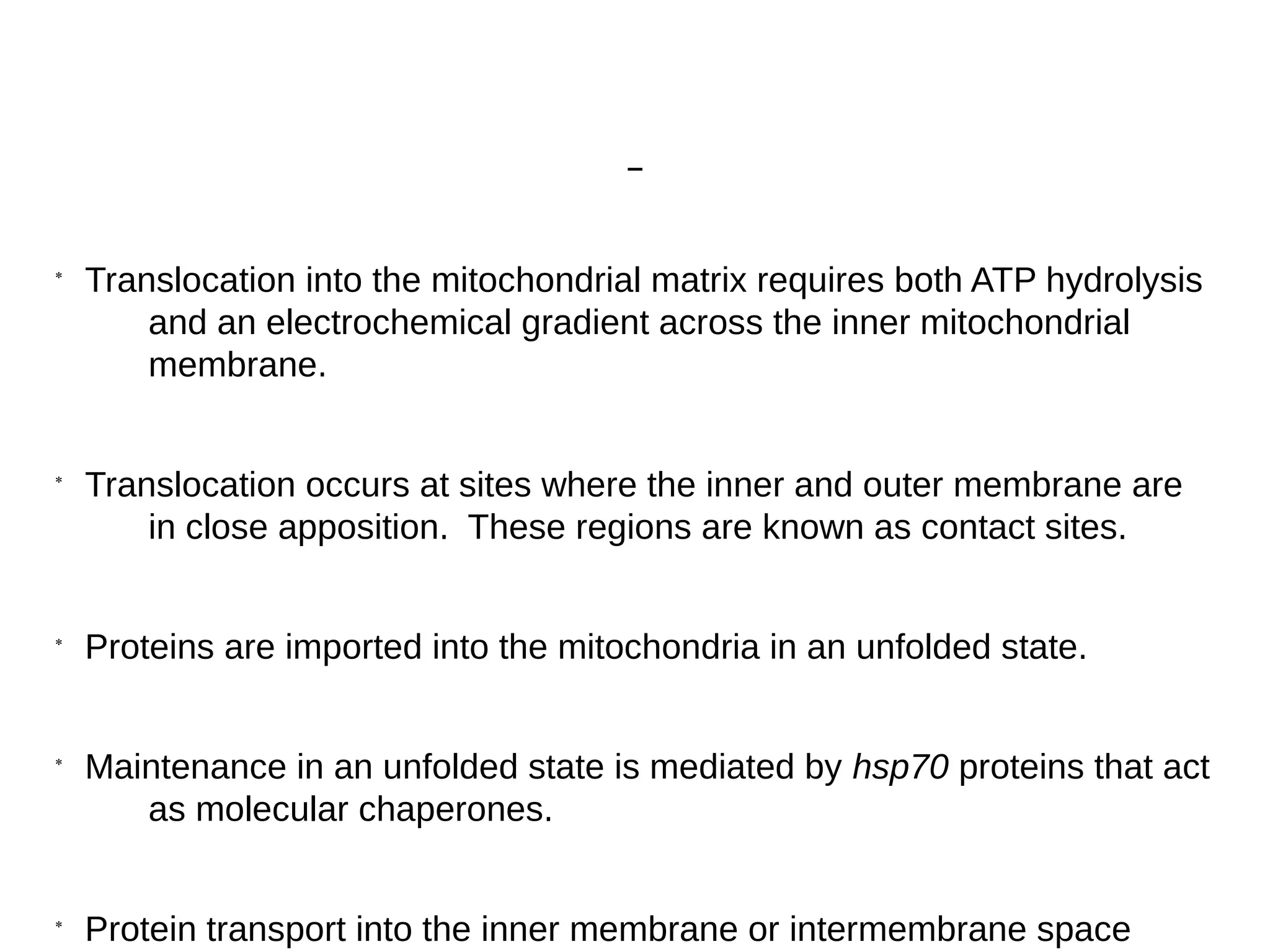
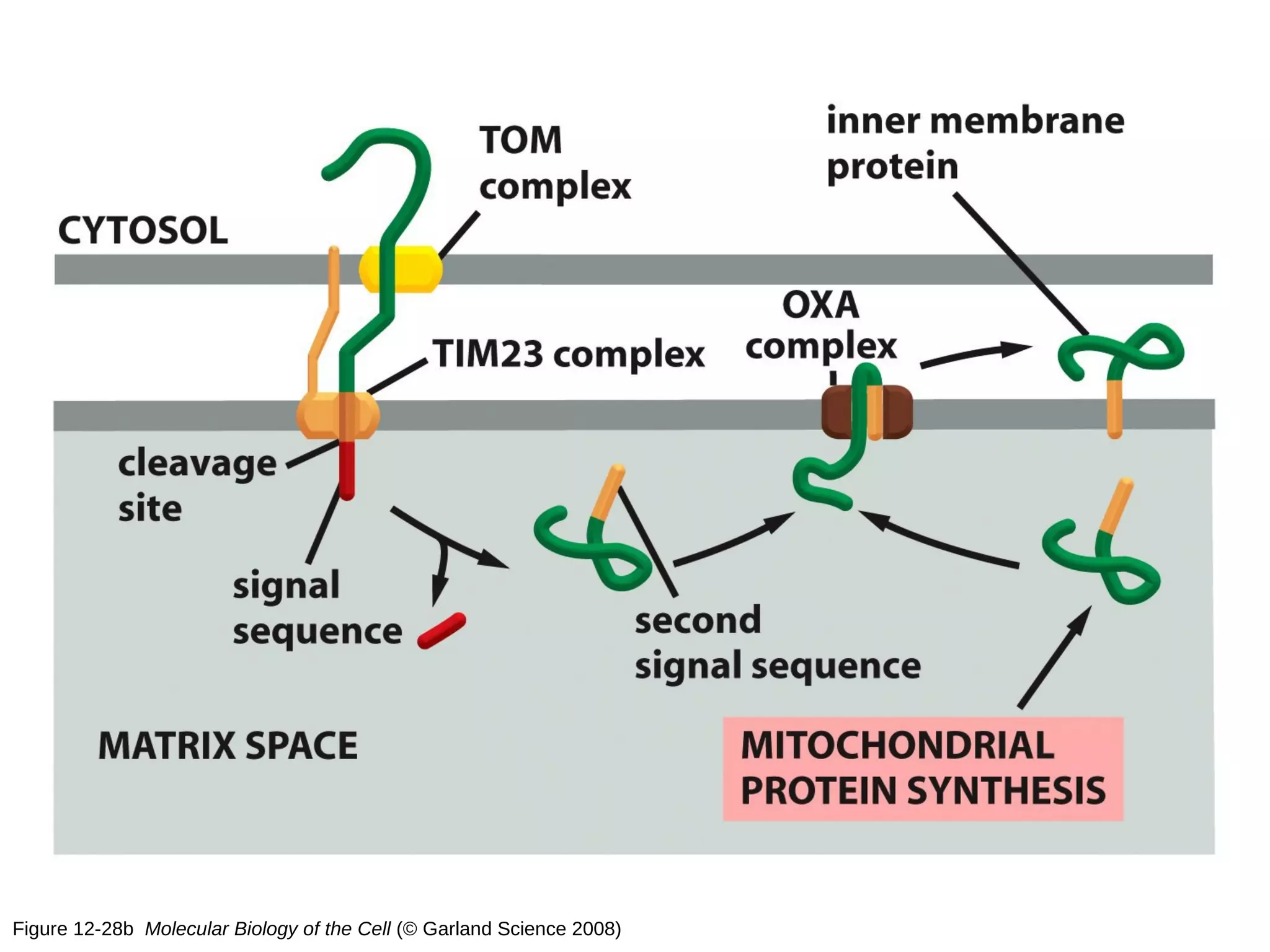
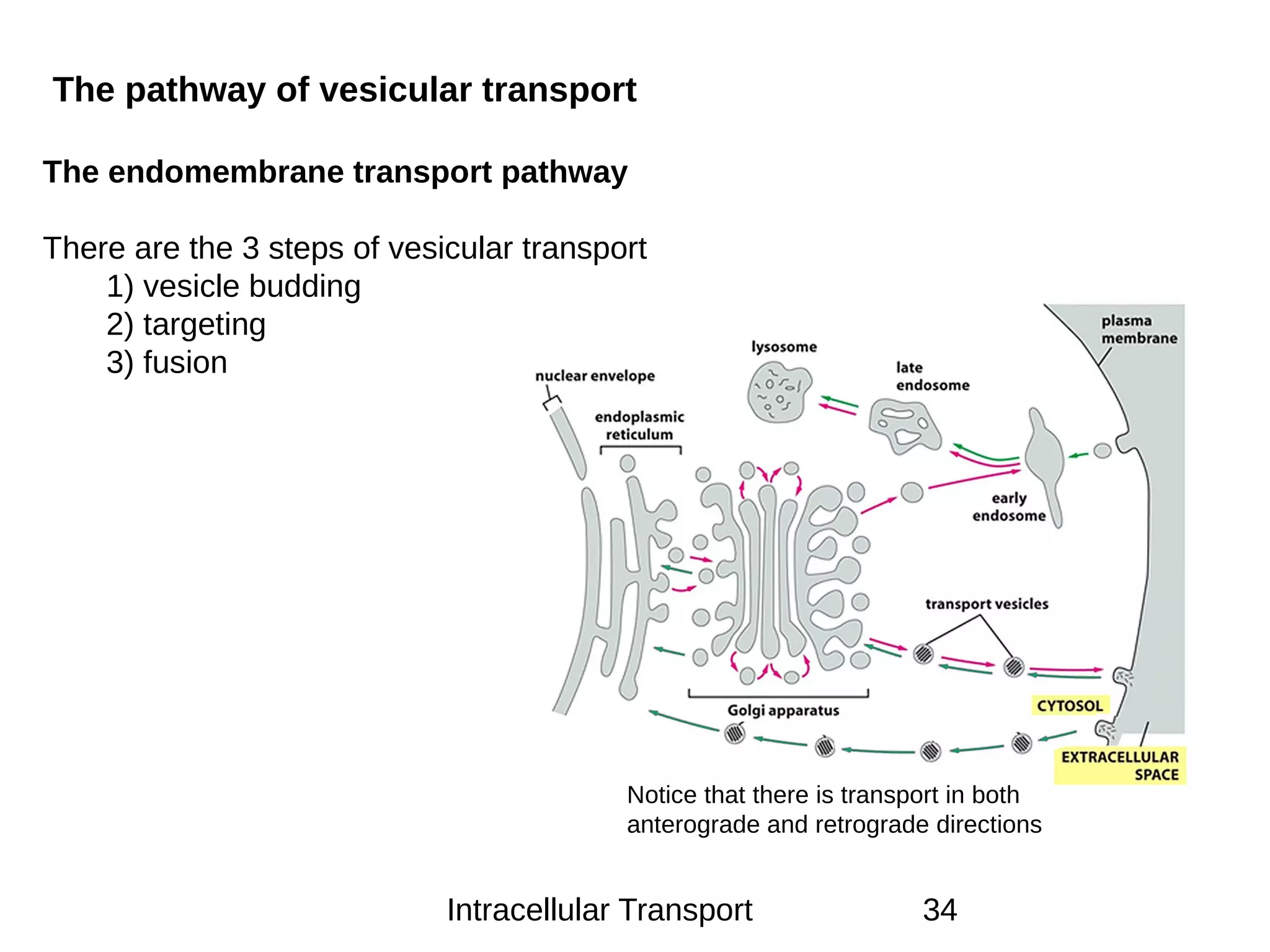
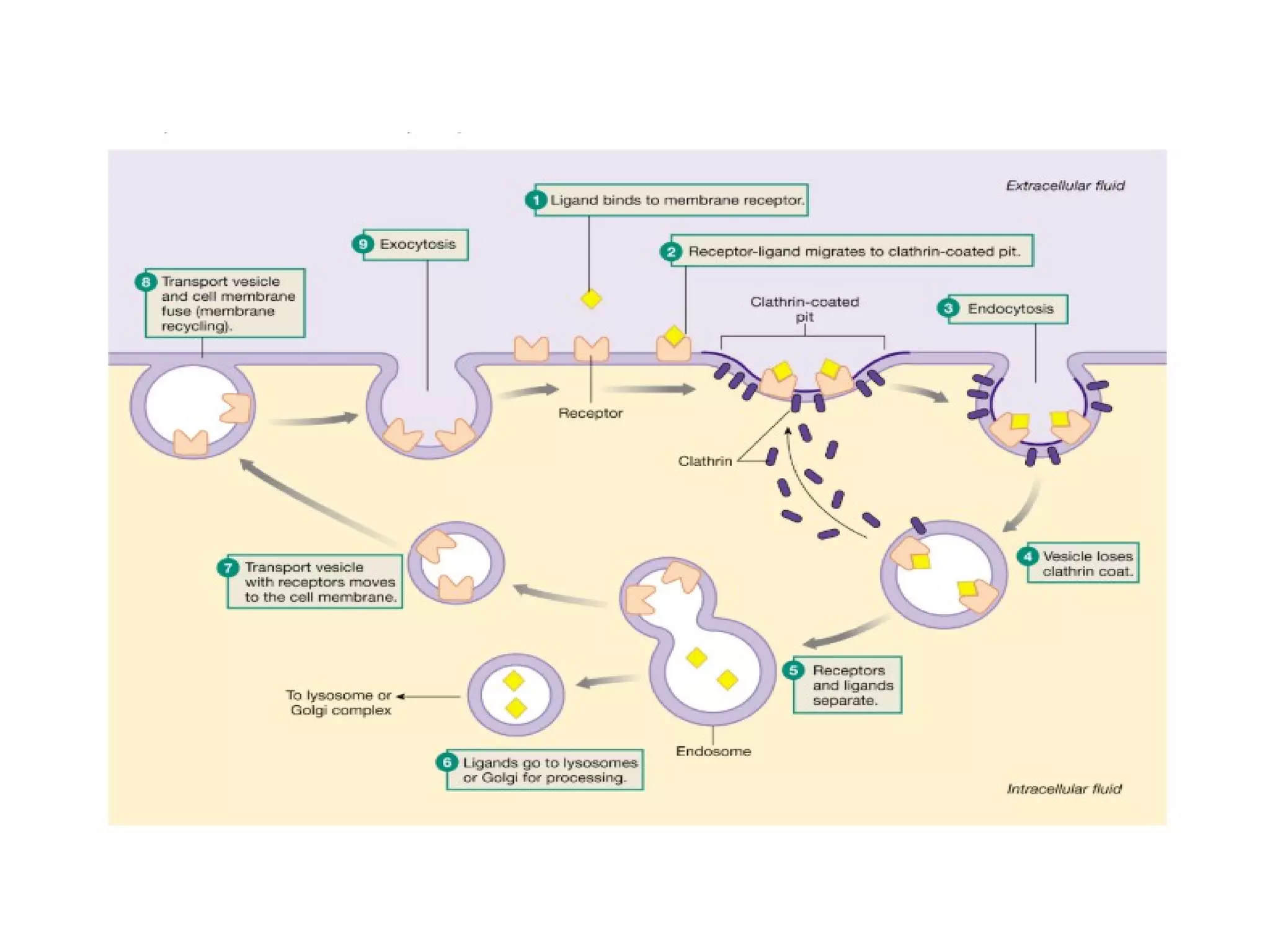
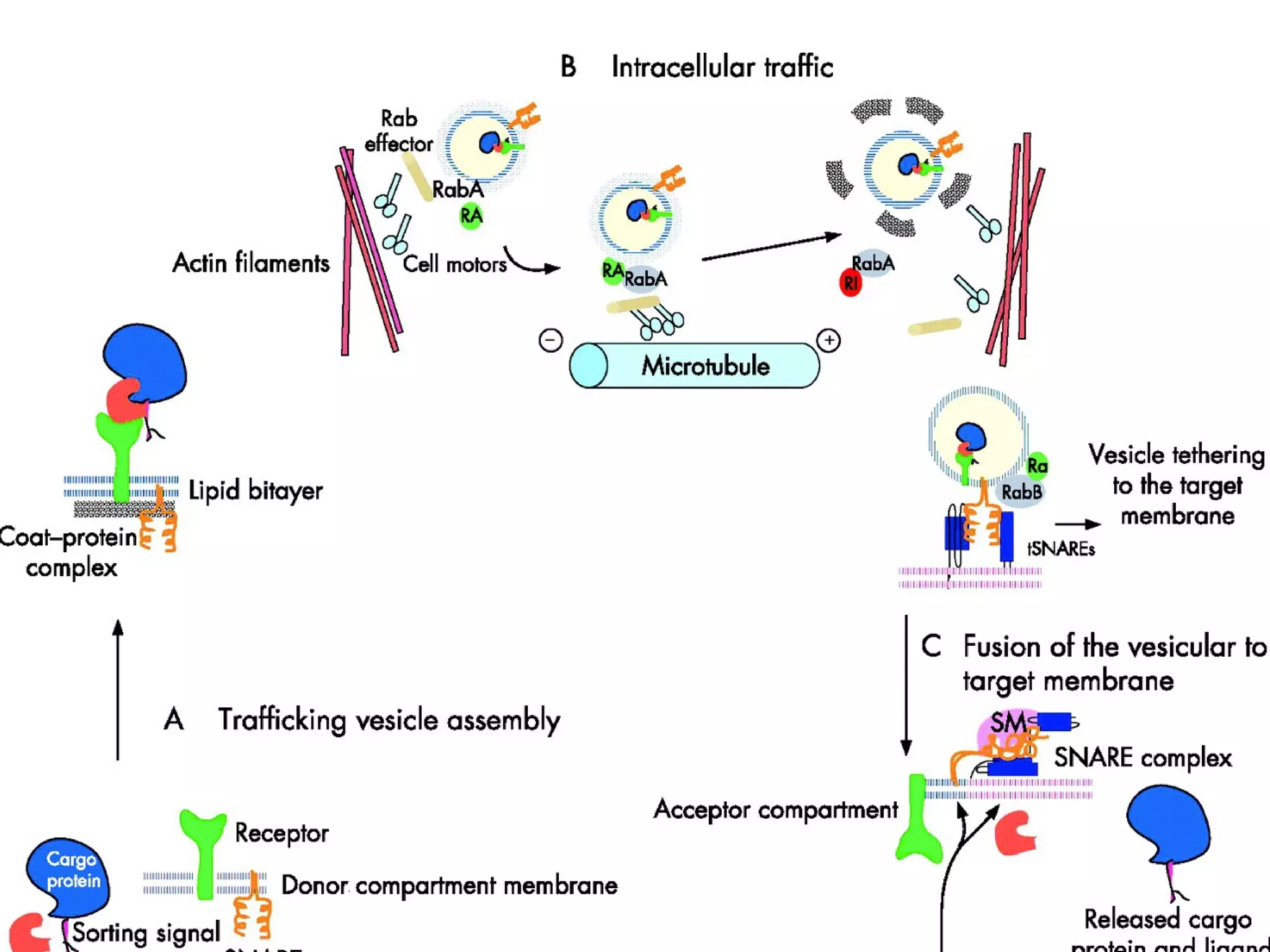
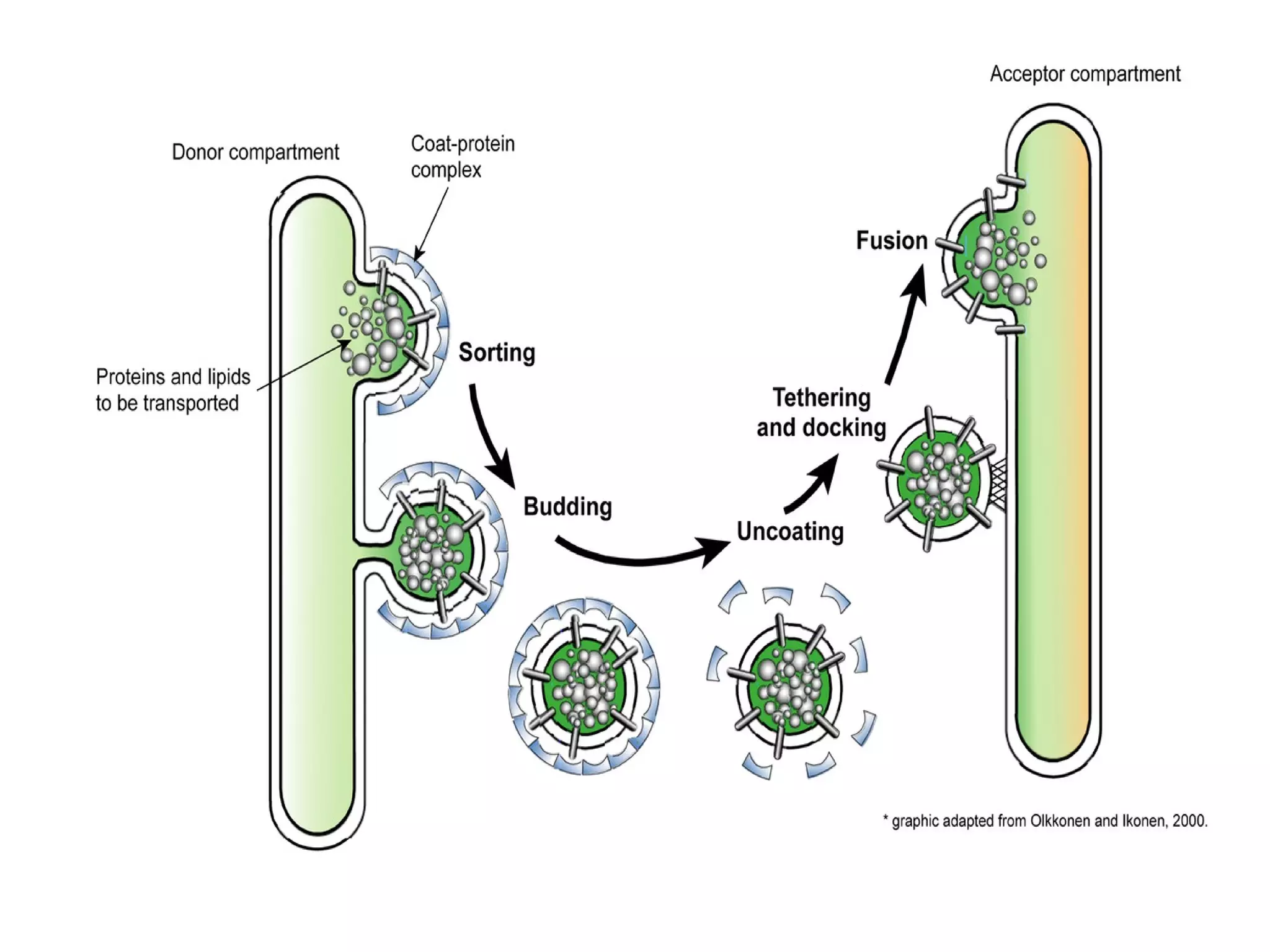
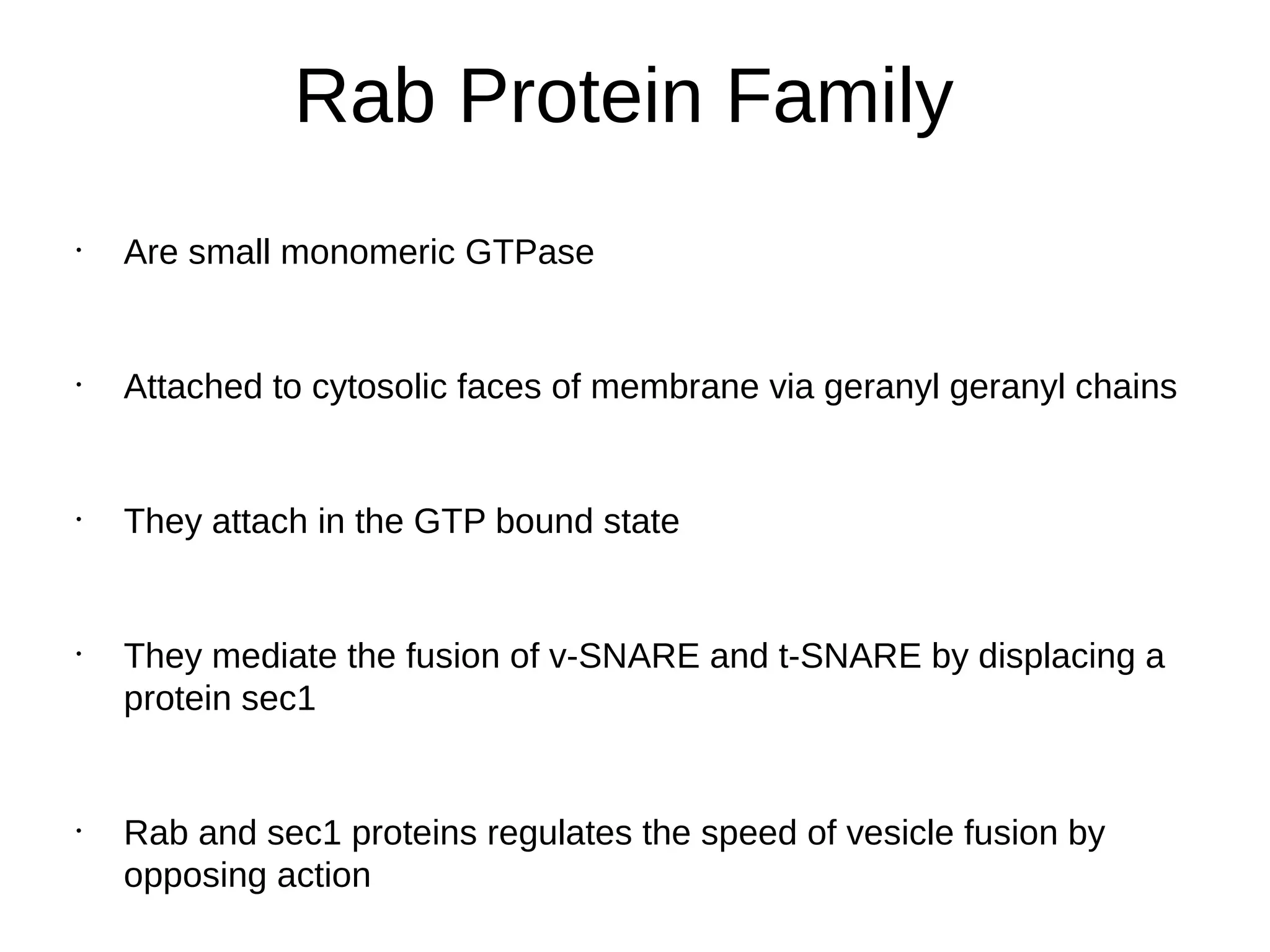
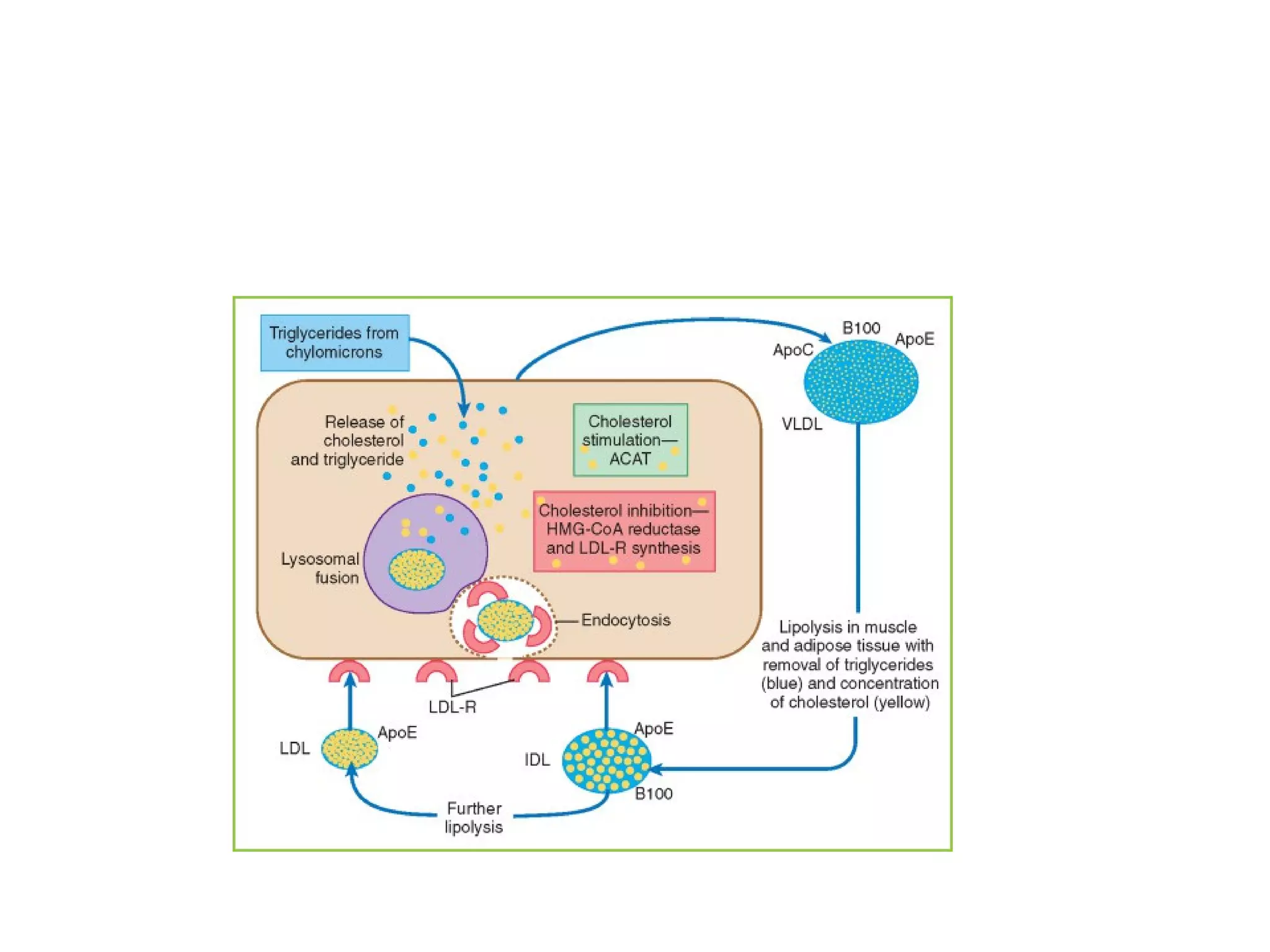
The document provides an in-depth overview of intracellular trafficking, focusing on protein sorting, transport mechanisms, and the roles of various cellular components such as nuclear pores and vesicles. It distinguishes between intracellular and intercellular transport and details the importance of the cytoskeleton and molecular motors in moving organelles and proteins within eukaryotic cells. Additionally, it discusses specific disorders related to intracellular transport and references various studies for further reading.