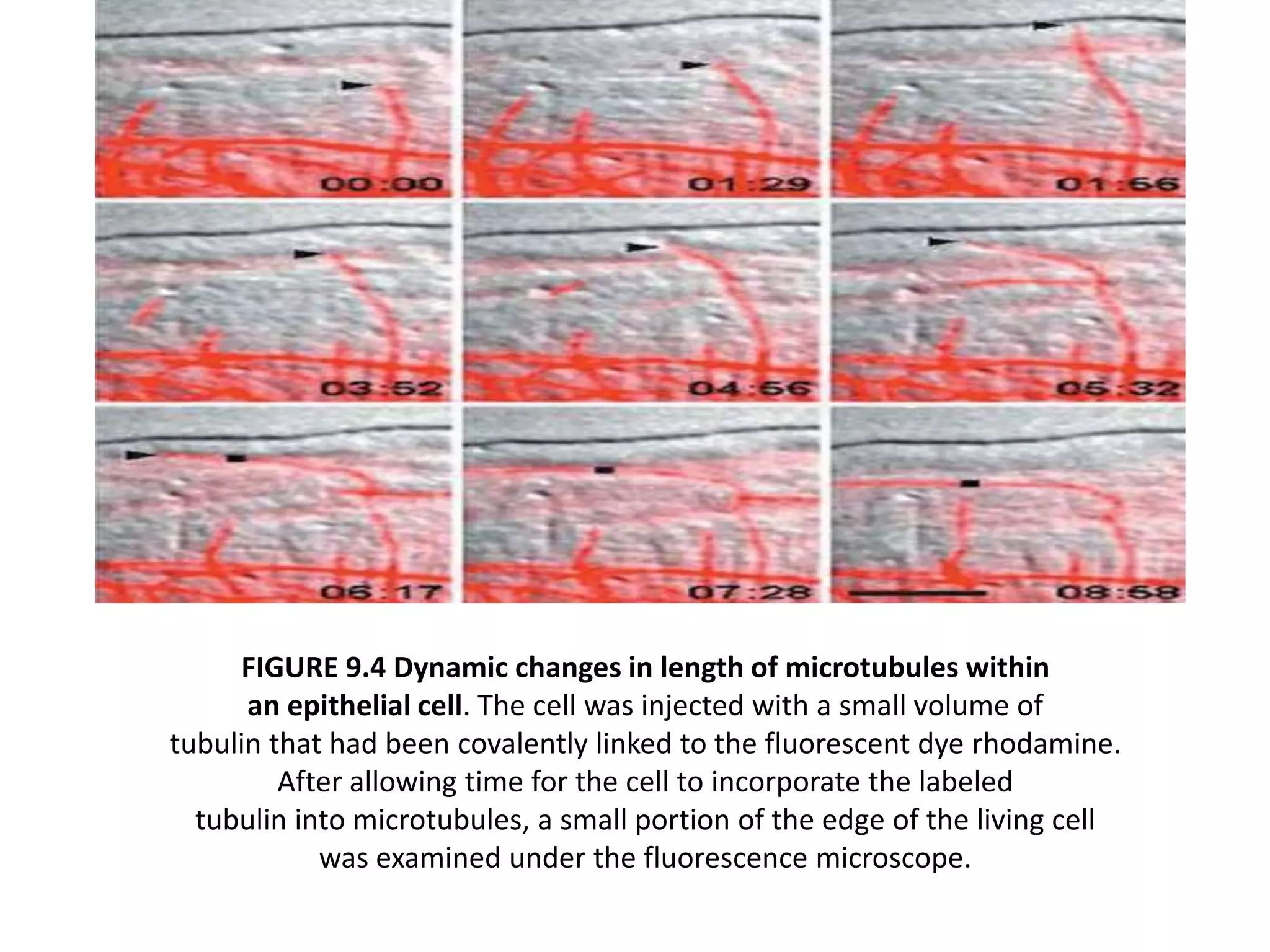
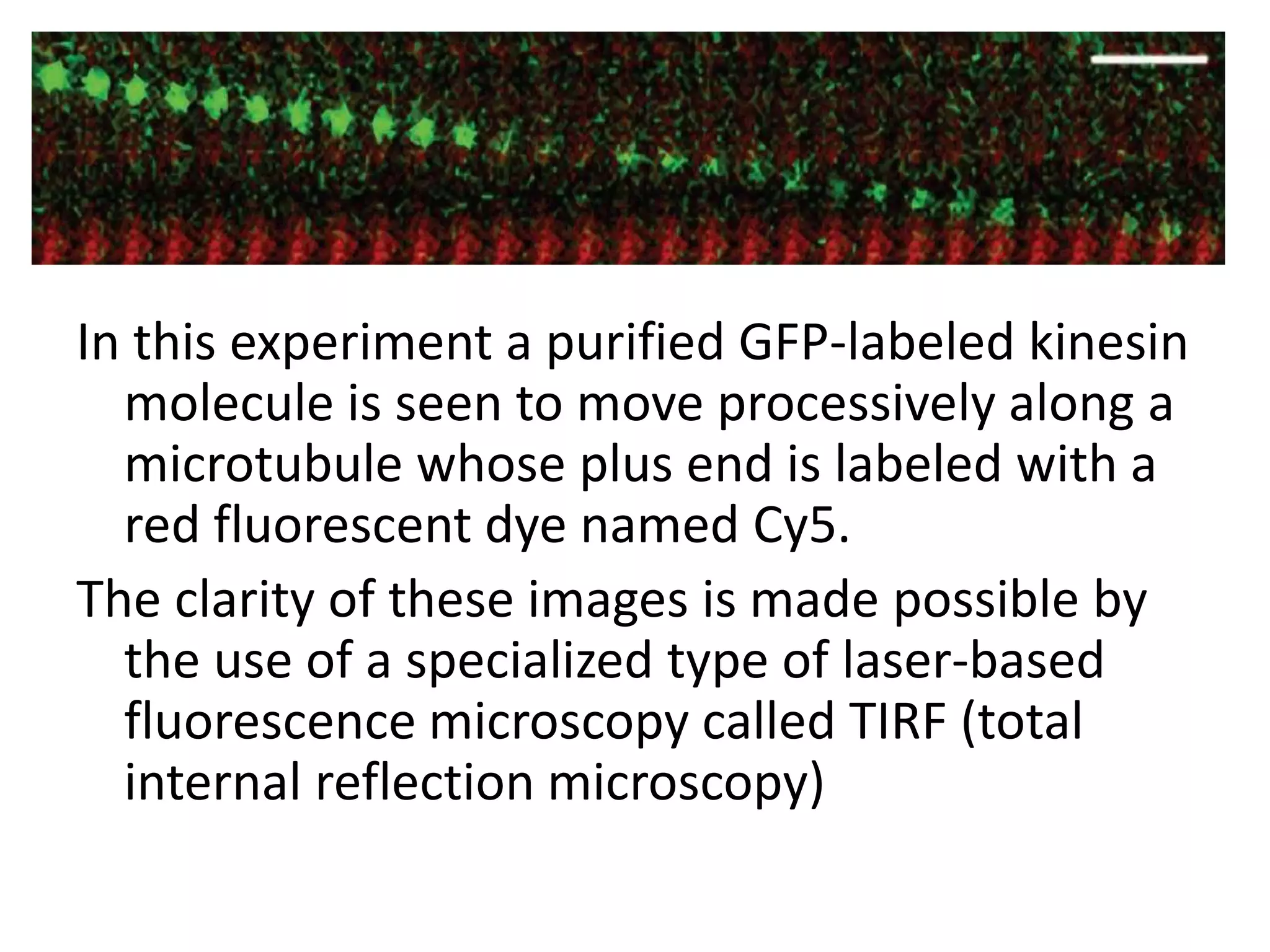
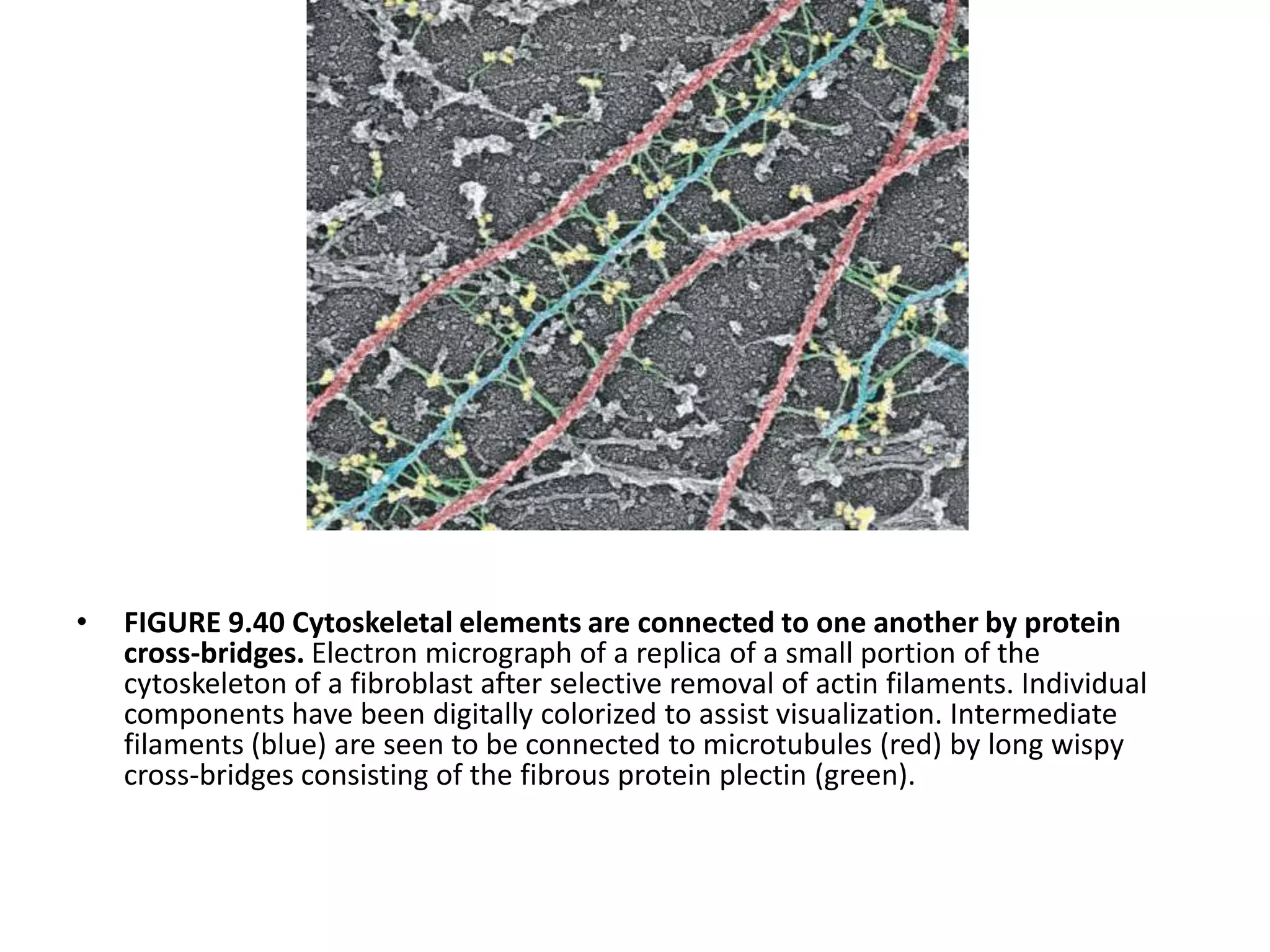
The cytoskeleton is a network of protein polymers that maintains cell shape and enables intracellular transport and movement. It consists of microtubules, microfilaments, and intermediate filaments. Microtubules are hollow tubes involved in structural support, transport, and cell organization. Microfilaments are thin filaments involved in motility and contractility. Intermediate filaments provide structural support. Motor proteins such as kinesins and dyneins interact with these cytoskeletal elements and convert chemical energy from ATP into mechanical force and movement.