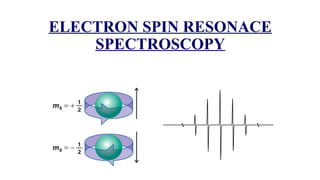
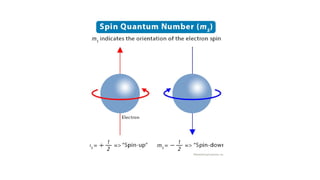
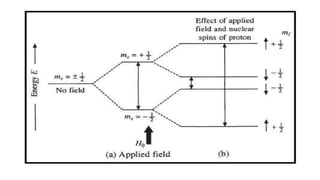
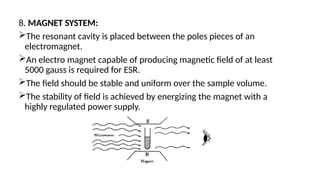
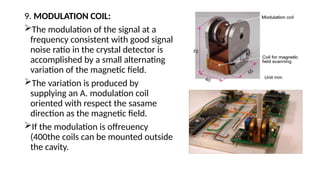
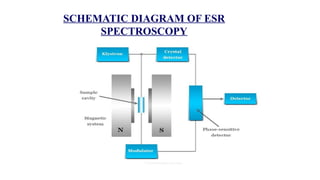
The document provides a comprehensive overview of Electron Spin Resonance (ESR) Spectroscopy, including its principles, instrumentation, and applications. ESR is used to study paramagnetic substances through the absorption of microwave radiation and is significant for analyzing metalloproteins and free radicals. Key components of ESR instrumentation are discussed, such as klystrons, wave guides, and magnet systems, illustrating their roles in the spectroscopy process.