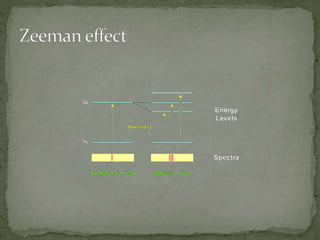
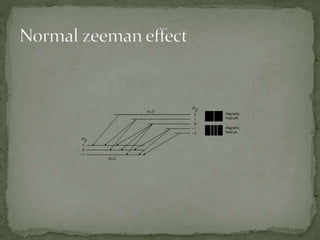
The Zeeman effect is the splitting of a spectral line into multiple spectral lines when in the presence of a magnetic field. It was first observed in 1896 by Dutch physicist Pieter Zeeman when he placed a sodium flame between magnetic poles and observed the broadening of spectral lines. Zeeman's discovery earned him the 1902 Nobel Prize in Physics. The pattern and amount of splitting provides information about the strength and presence of the magnetic field.