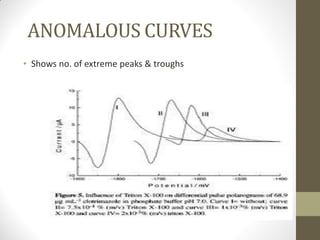
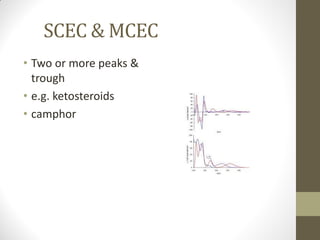
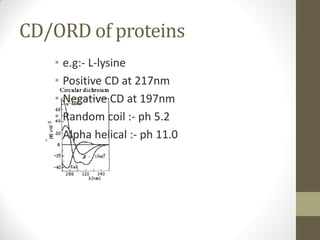
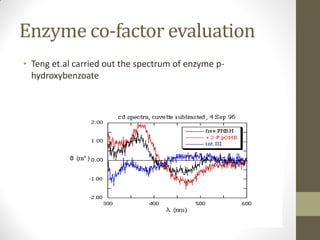
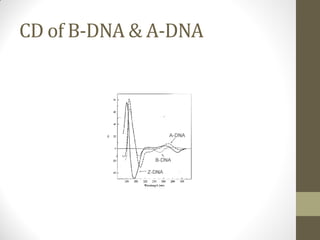
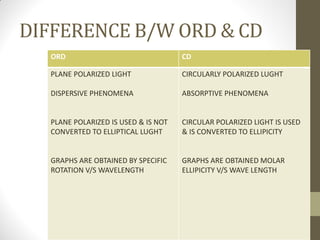
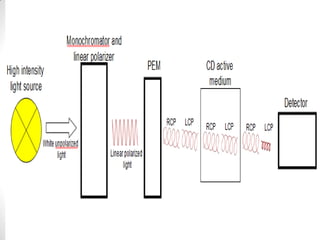
This document discusses optical rotatory dispersion (ORD) and circular dichroism (CD). ORD measures the change in specific rotation of plane-polarized light with wavelength for optically active compounds. CD measures the difference in absorption of left and right circularly polarized light. Key differences are that ORD uses plane-polarized light while CD uses circularly polarized light. ORD graphs plot specific rotation versus wavelength while CD graphs plot molar ellipticity versus wavelength. Both techniques provide information about molecular structure and can be used to analyze proteins, nucleic acids, and other biomolecules.






![SPECIFIC ROTATION
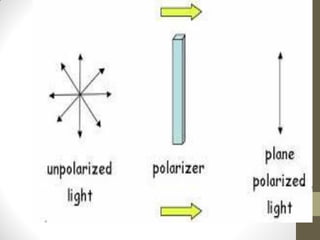
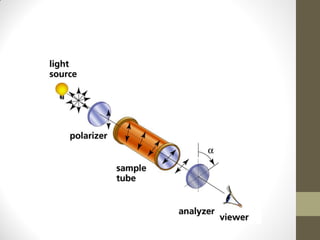
• The specific rotation ([α]) is an intensive property of
a chemical compound, defined as the change in orientation of
the plane of linearly polarized light as this light passes through
a sample with a path length of 1 decimeter and a sample
concentration of 1 gram per 1 millilitre
• It is denoted by [α]](https://image.slidesharecdn.com/ordcd-180519043318/85/OPTICAL-ROTATORY-DISPERSION-CIRCULAR-DICHORISM-ORD-CD-9-320.jpg)