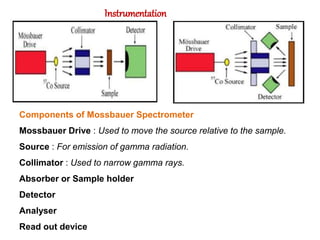
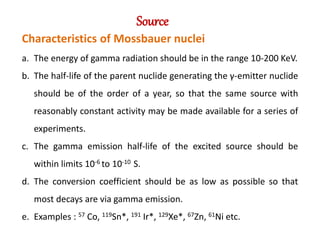
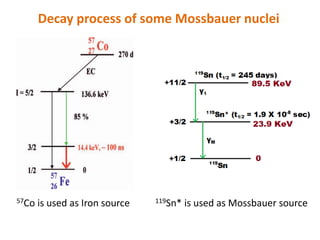
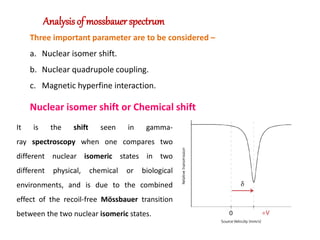
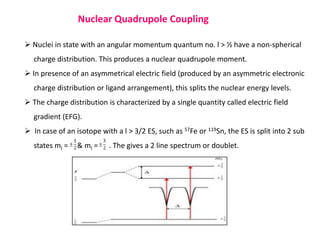
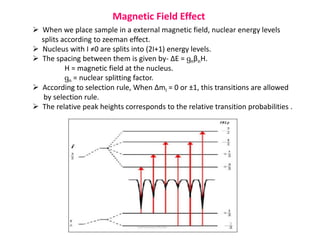
Mossbauer spectroscopy involves the resonant absorption and emission of gamma rays between atomic nuclei bound in a solid material. It can provide information about the chemical and electronic environment of atomic nuclei. The document discusses the basic principles, instrumentation, and analysis of Mossbauer spectroscopy. Key applications include determining chemical shifts, quadrupole splitting, magnetic properties, and using these parameters to study chemical bonding, structure, and biochemical systems.


![The source and sample nuclei used in Mossbauer spectroscopy is
same, but the reabsorption of emitted γ- ray were not taking place
at the same frequency, this is due to –
i] Nuclear recoil phenomenon.
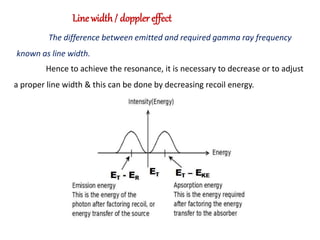
ii] Doppler effect / Line width.](https://image.slidesharecdn.com/mossbauerspectroscopy-210921105440/85/Mossbauer-spectroscopy-4-320.jpg)