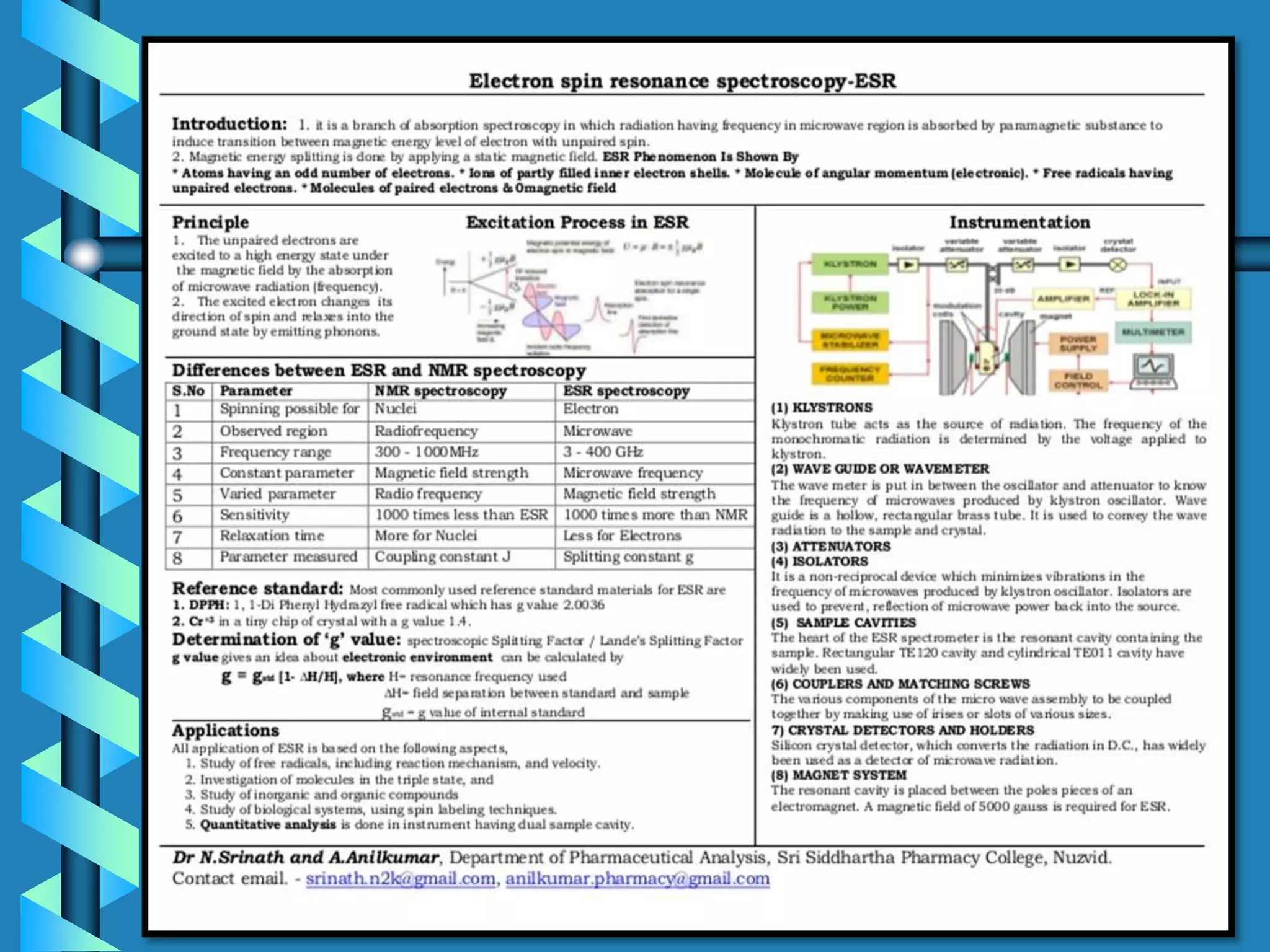
Electron spin resonance (ESR) spectroscopy involves exposing paramagnetic substances containing unpaired electrons to microwave radiation, causing transitions between the electron spin energy levels. ESR provides information about unpaired electrons and their chemical environment. The ESR spectrum of hydrogen atom appears as a doublet due to interaction between the unpaired electron and nuclear spin of hydrogen. More complex spectra result from interactions between unpaired electrons and multiple nuclear spins. ESR is used to study paramagnetic species and identify unpaired electrons in compounds.