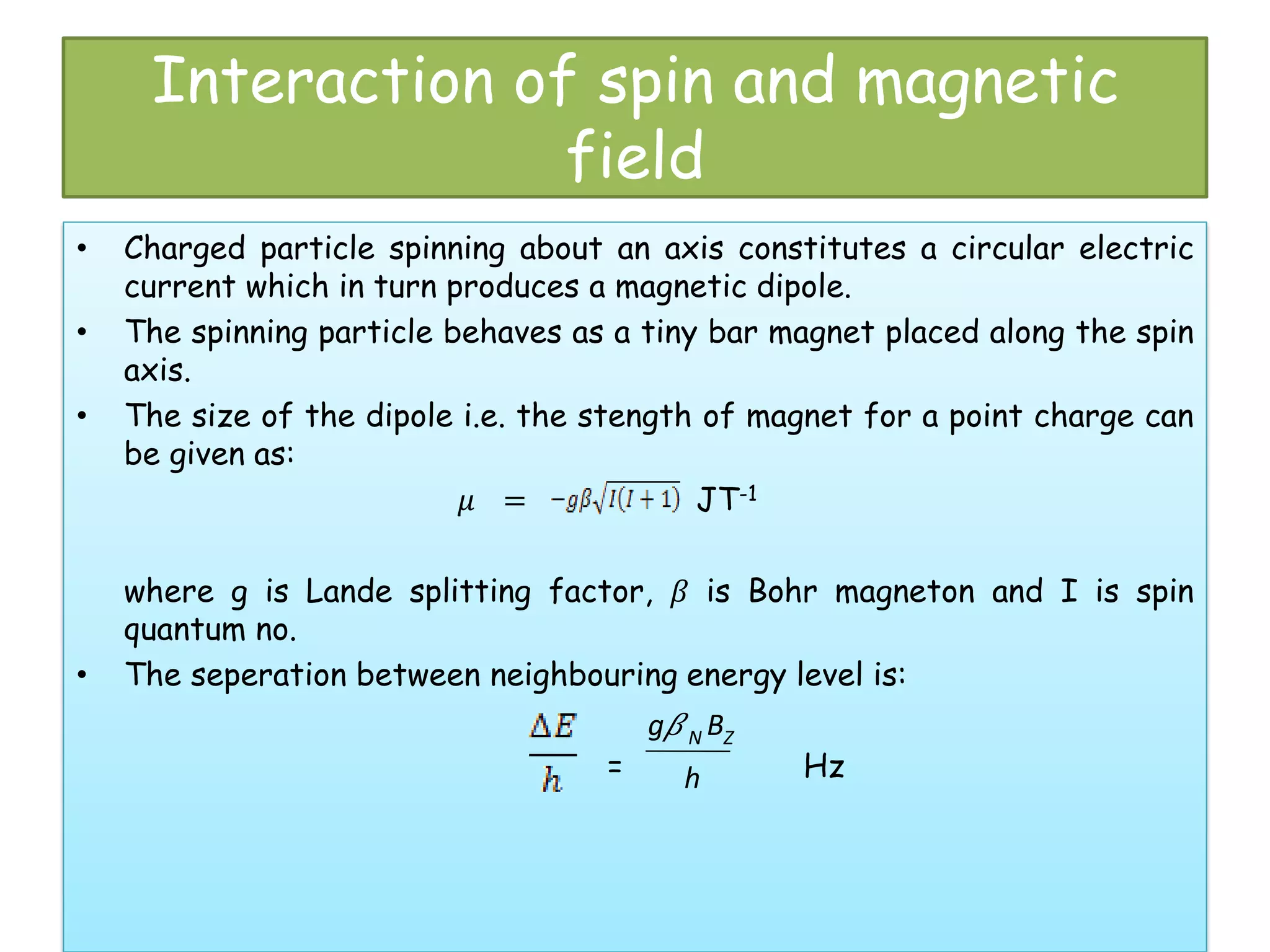
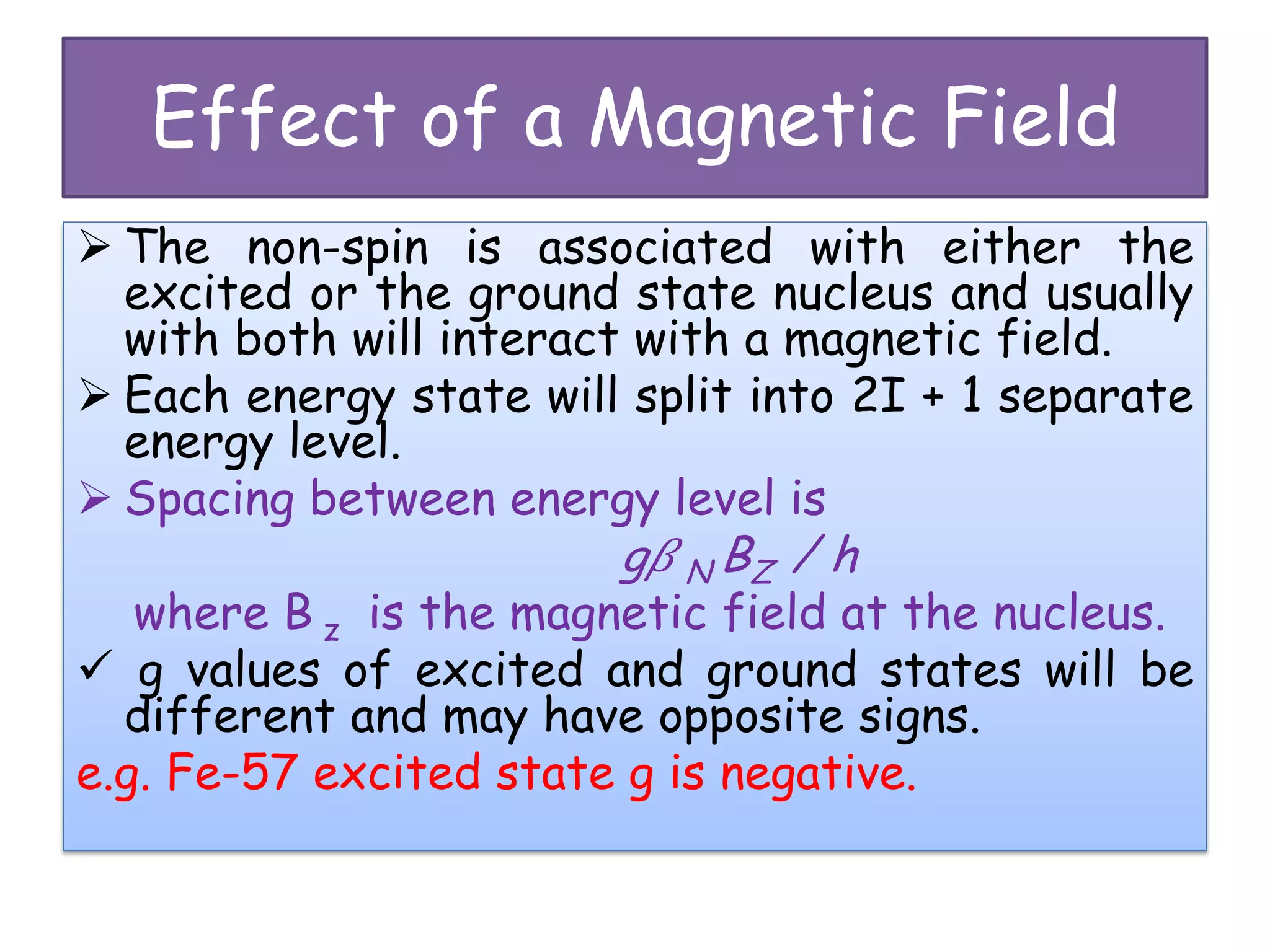
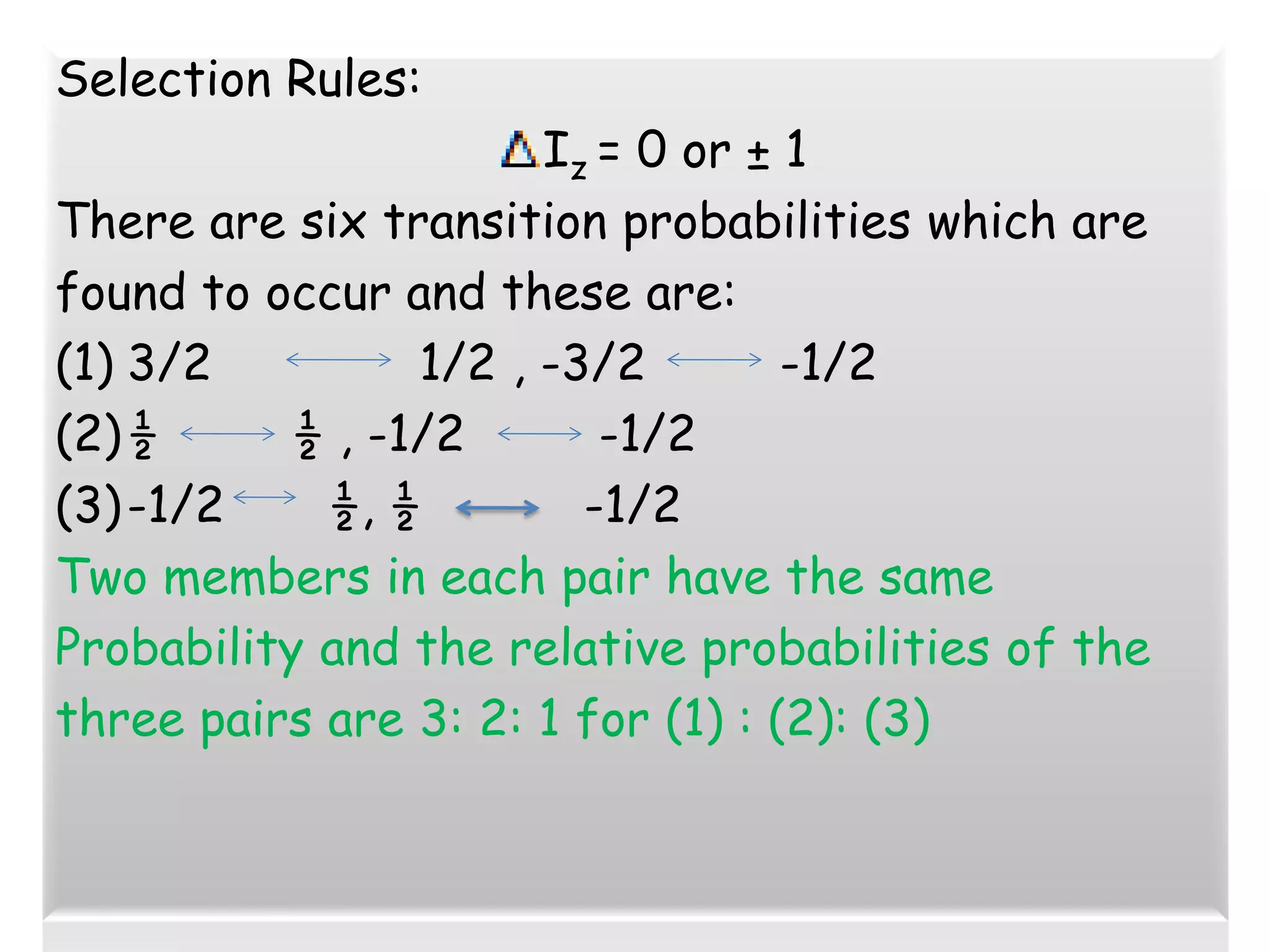
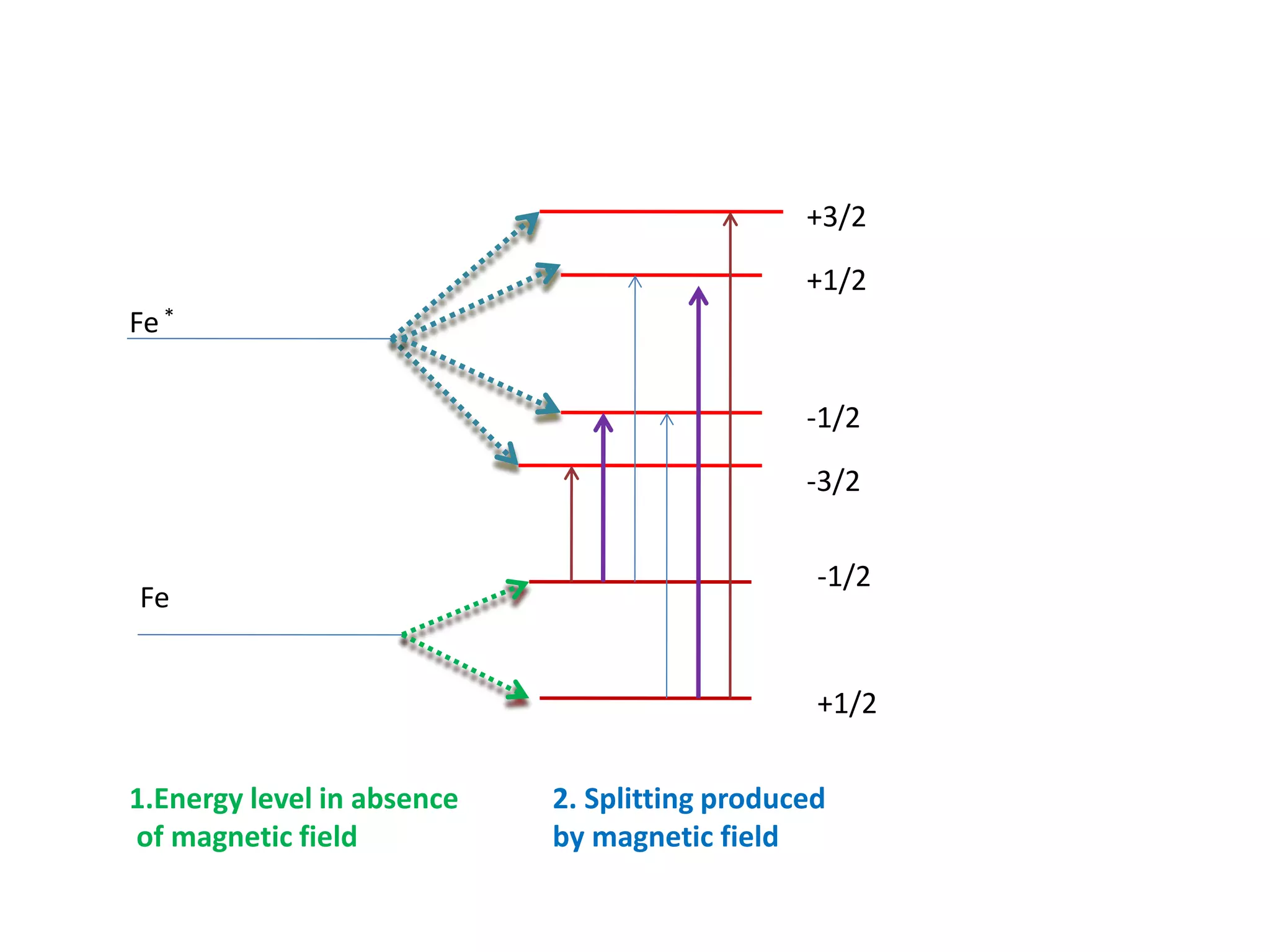
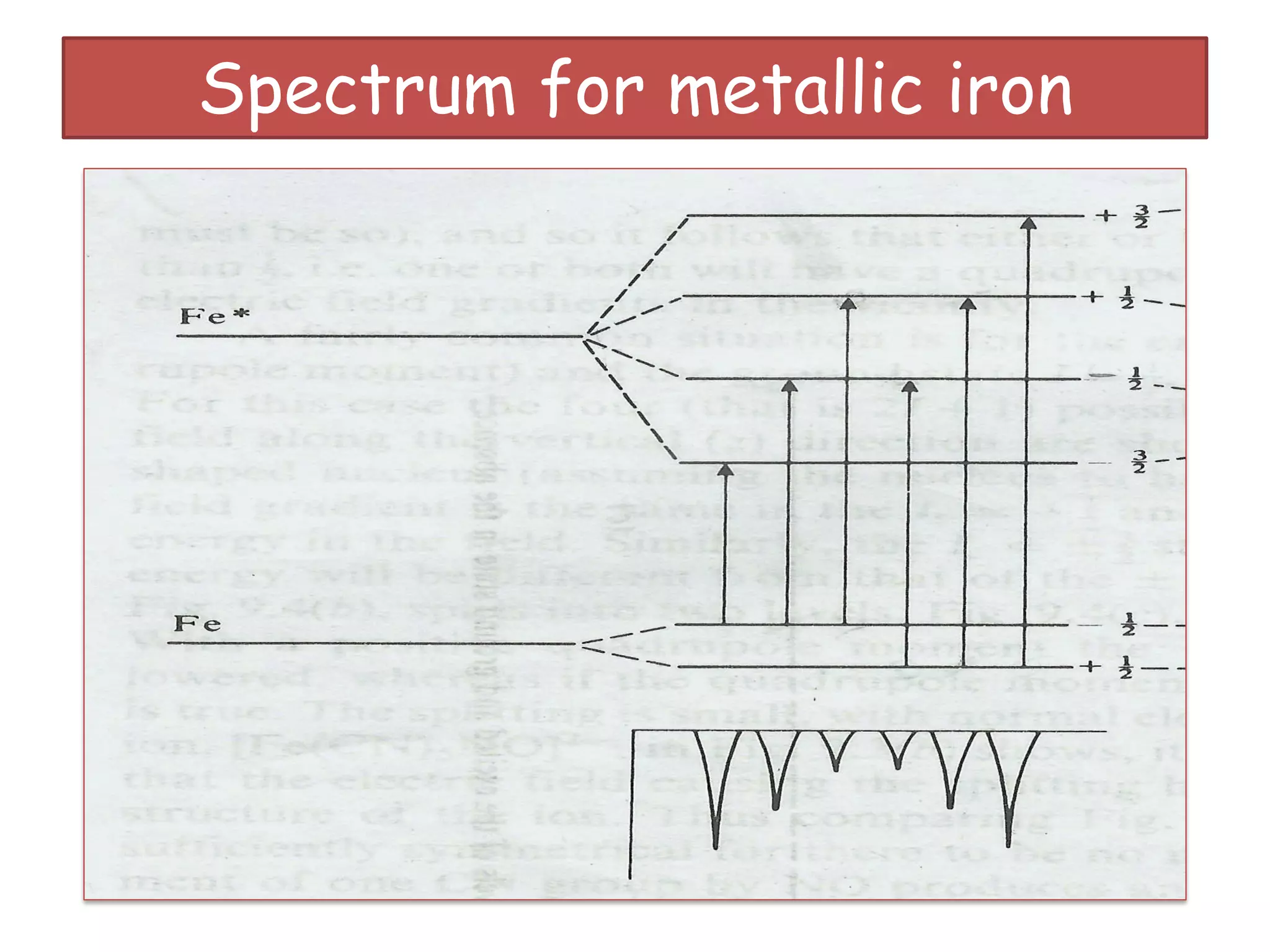
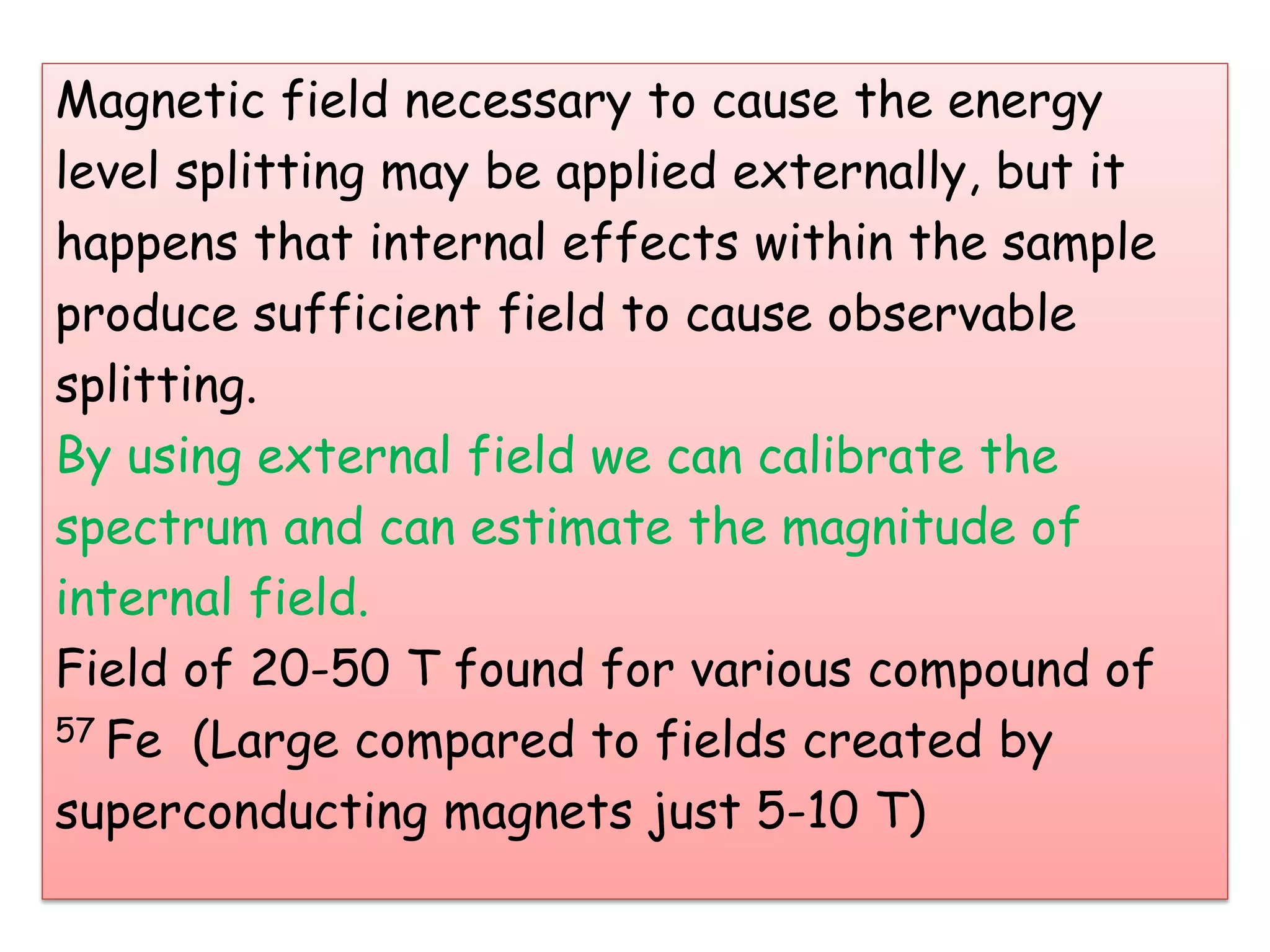
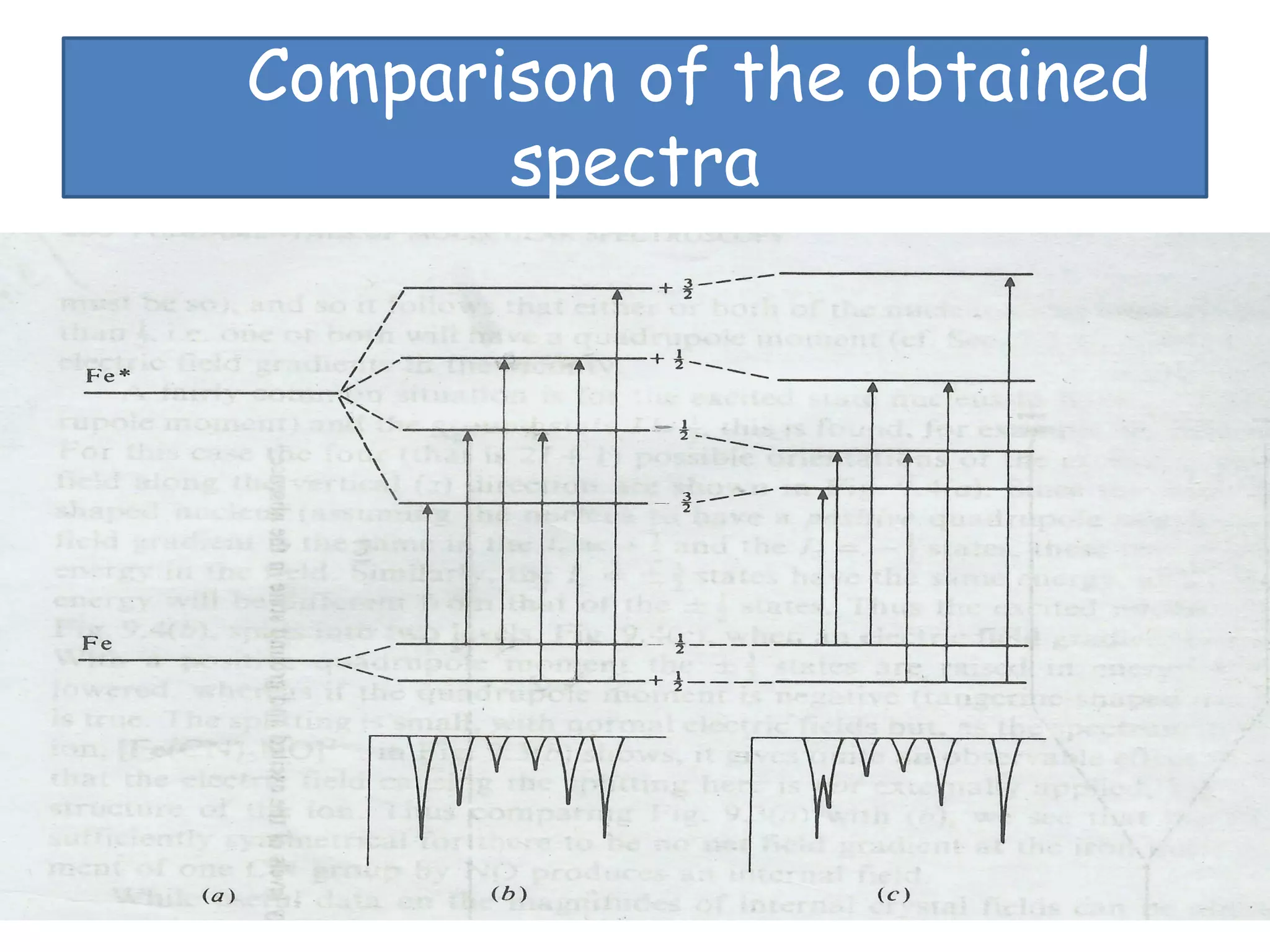
Mössbauer spectroscopy involves the recoil-free emission and absorption of gamma rays within atomic nuclei. It can provide information about the chemical and physical properties of materials. When nuclei decay, energy is emitted. In the presence of a magnetic field, the nuclear energy levels will split according to the spin and magnetic moment. This splitting produces a characteristic spectrum that can reveal properties like internal magnetic fields. The document discusses the principles, interactions with magnetic fields, selection rules, and examples of spectra for different materials like iron and iron difluoride.