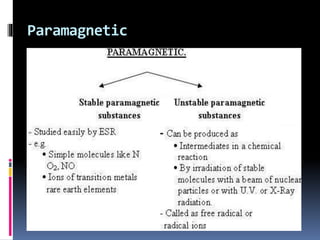
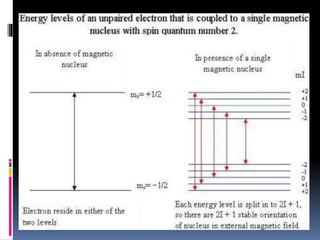
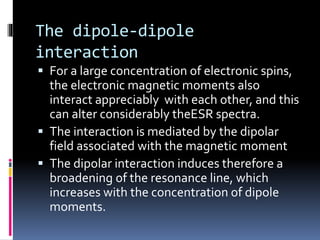
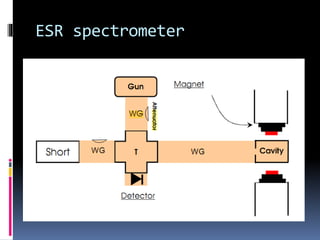
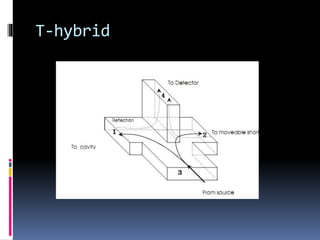
Electron spin resonance spectroscopy (ESR) involves using microwave radiation to induce transitions between the magnetic energy levels of unpaired electrons in paramagnetic molecules. It provides information about unpaired electrons and free radicals. The key components of an ESR spectrometer are a microwave source, waveguide, cavity, and detector. ESR has applications in studying free radicals, molecules in the triple state, and inorganic compounds. It is used analytically to detect trace ions and estimate oxidation states, and in biological systems to study metabolic activity, diseases, and photosynthesis.