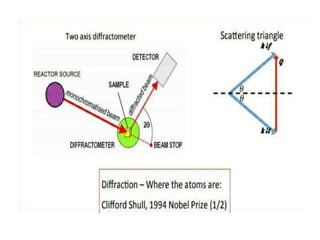
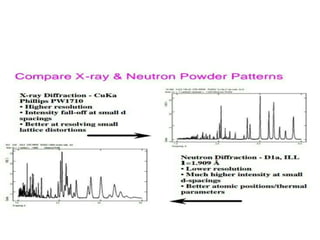
The document discusses neutron diffraction, a technique used for determining atomic and magnetic structures of materials, and compares it with X-ray diffraction (XRD). It outlines the principles, instrumentation, and various applications of neutron diffraction while highlighting its advantages over XRD, such as better detection of light elements and magnetic structures. The future of neutron diffraction is promising, with increasing demand in material structure research and advancements aimed at exploring new technologies for electrical energy storage.