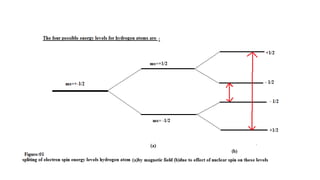
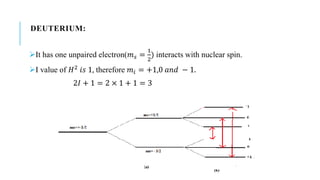
Hyperfine splitting occurs due to the interaction between an electron's spin and the nucleus' spin. This interaction causes each electron spin state to split into 2I+1 levels, where I is the nuclear spin quantum number. As examples, the document discusses the hyperfine splitting in hydrogen, where the nuclear spin is 1/2, and deuterium, where the nuclear spin is 1. Hyperfine splitting has applications in radio astronomy, nuclear technology such as laser isotope separation, and atomic clocks.