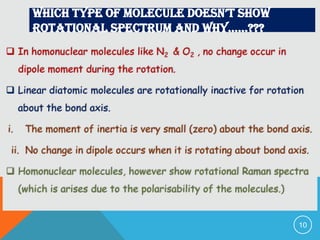
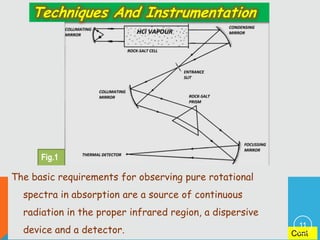
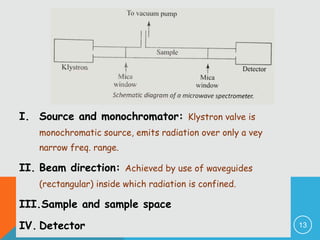
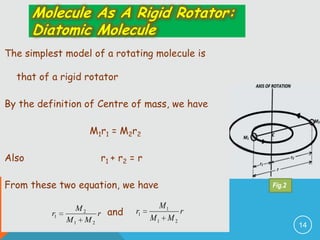
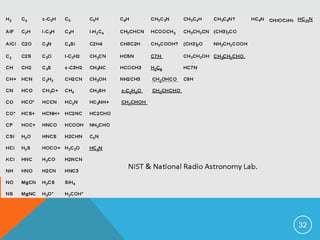
The document provides information on rotational spectroscopy and the rotational spectra of molecules. It discusses key topics like:
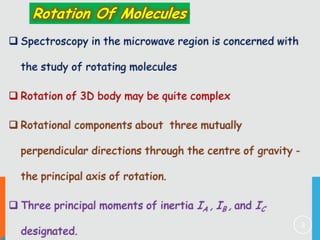
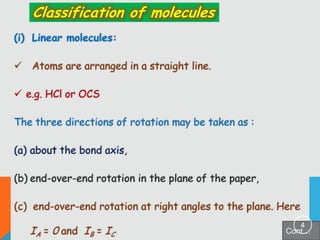
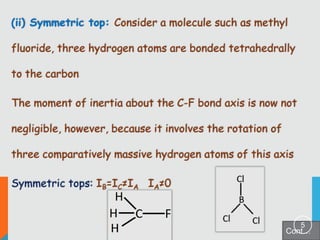
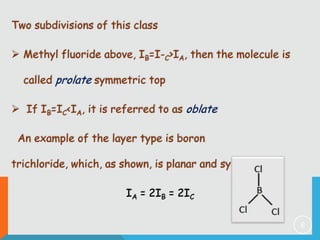
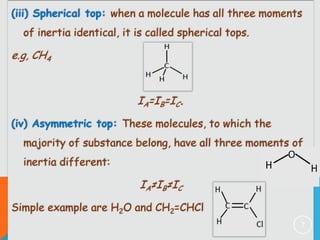
1) Classification of molecules as linear, symmetric top, spherical top, and asymmetric top based on their moments of inertia.
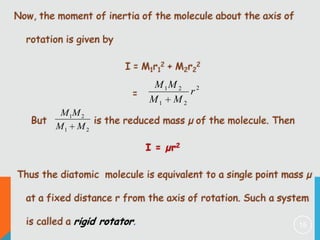
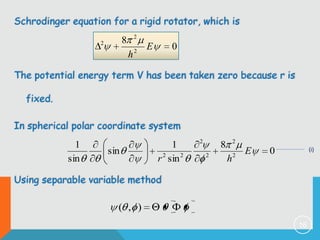
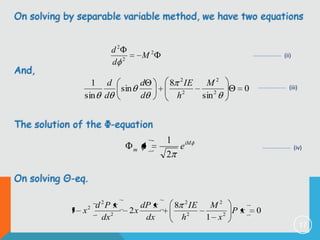
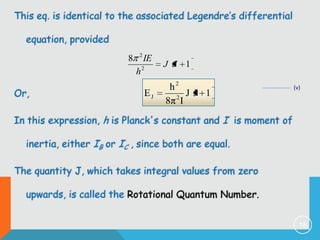
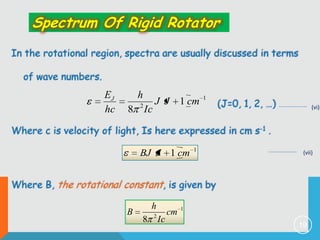
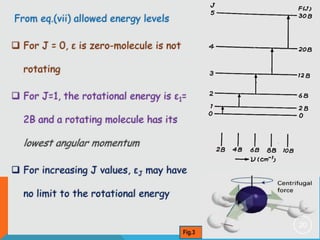
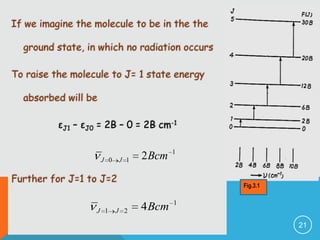
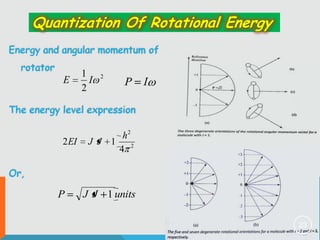
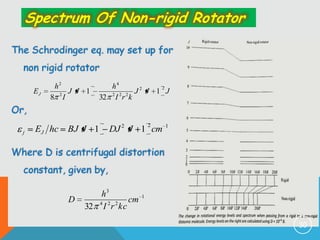
2) The rigid rotor model and how it leads to quantized rotational energy levels expressed by the rotational constant B.
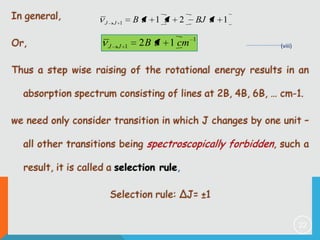
3) The selection rule for rotational transitions of ΔJ = ±1, which results in a series of equally spaced spectral lines.
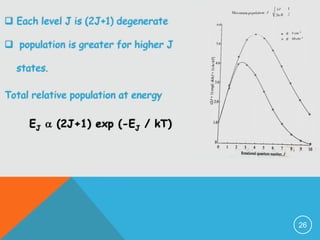
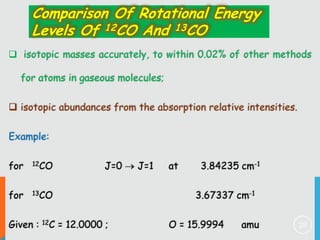
4) Factors that determine the intensity of rotational lines, including Boltzmann distribution of molecular populations and degeneracy of energy levels.