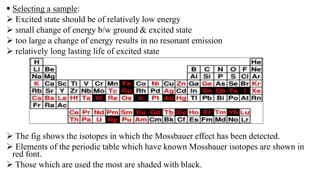
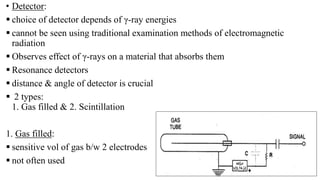
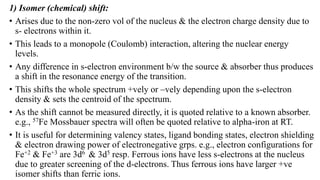
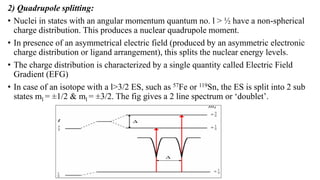
Mossbauer spectroscopy is a technique based on the resonance fluorescence of gamma radiation, allowing the recoil-less emission and absorption of gamma rays by atomic nuclei in solids. It involves essential principles like recoil energy, Doppler shift, and hyperfine interactions, which include isomer shift, quadrupole splitting, and magnetic splitting. The method is widely applied in fields like inorganic chemistry and biology to study chemical bonding and various nuclear properties.