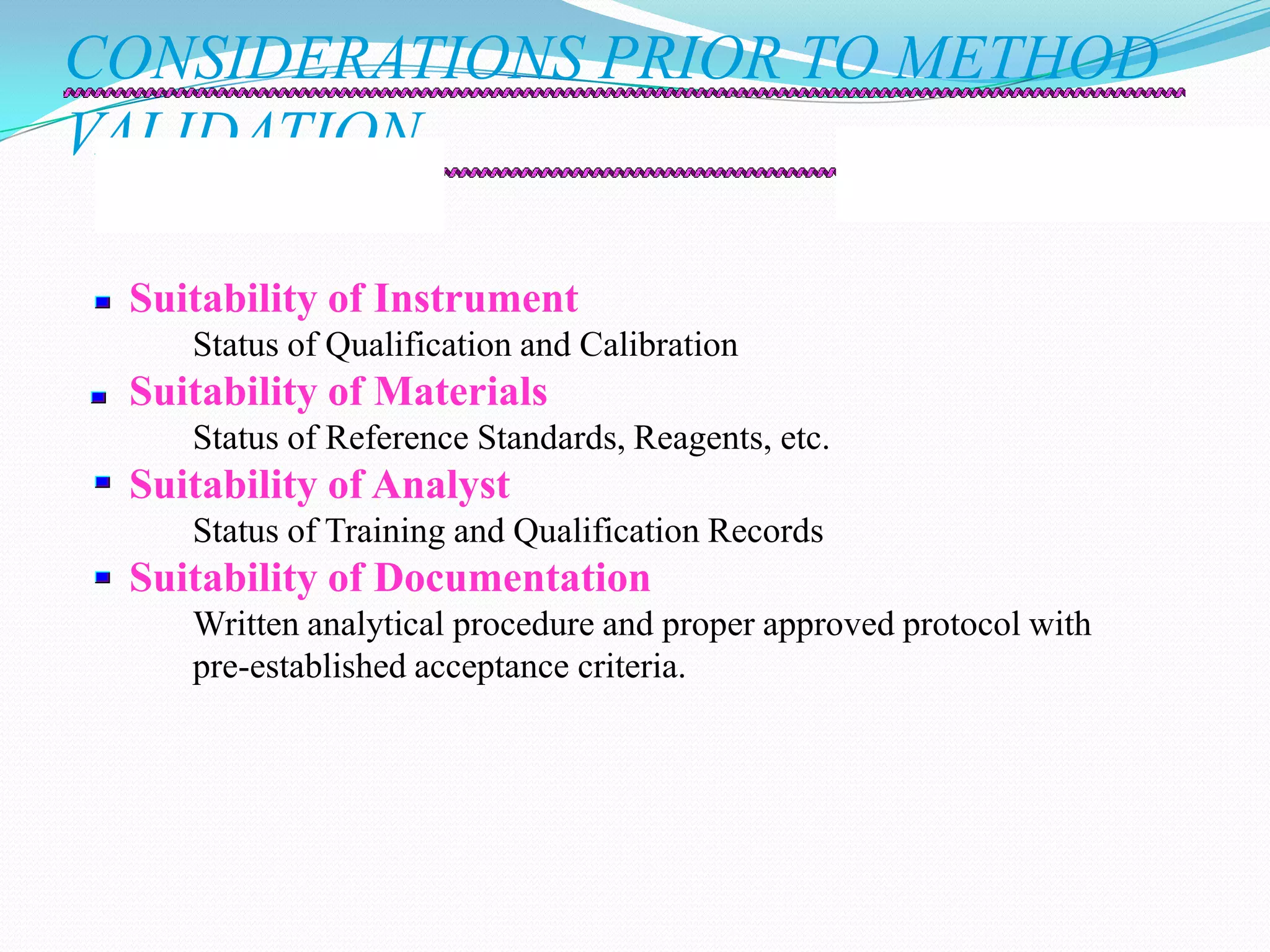
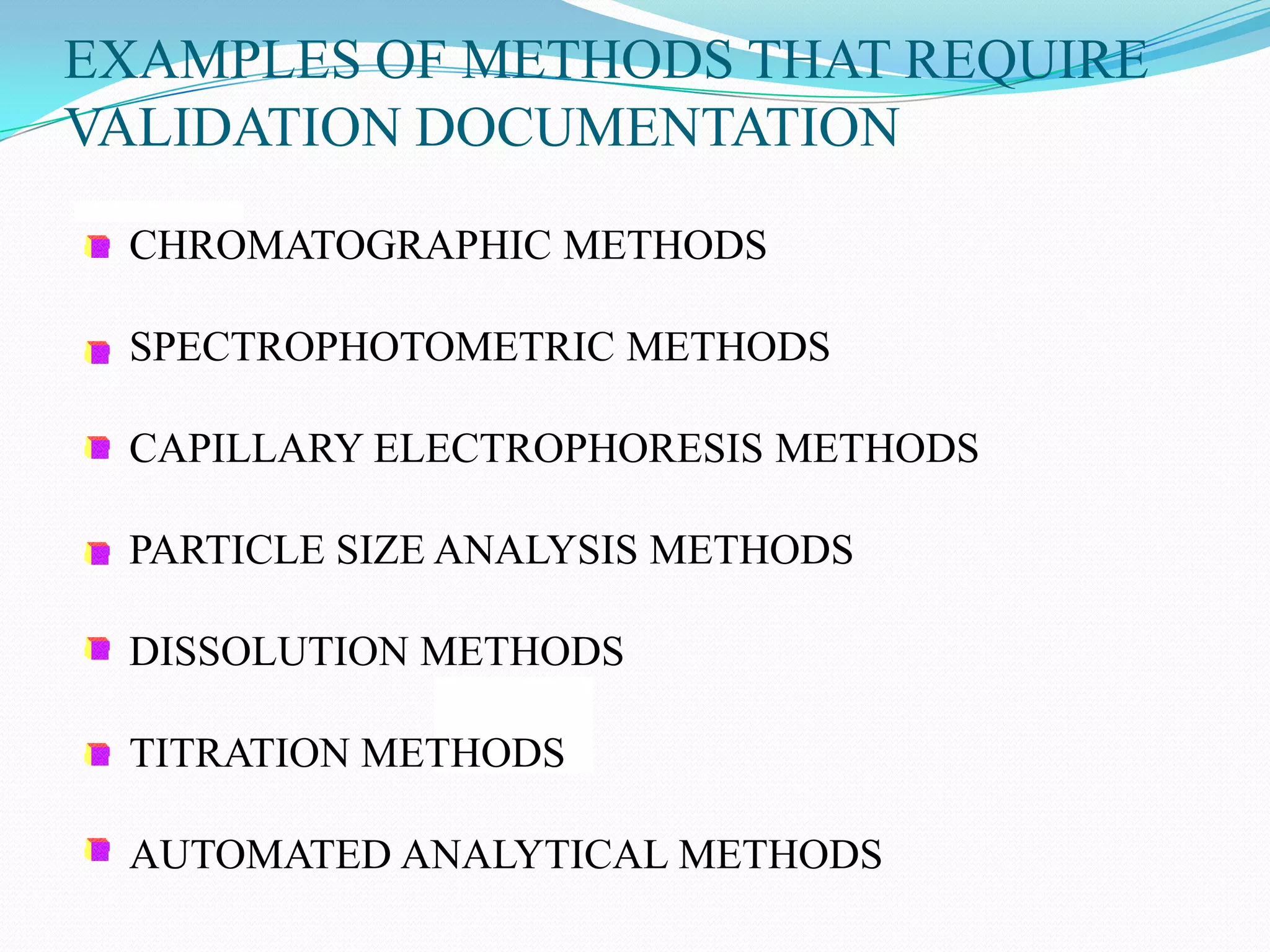
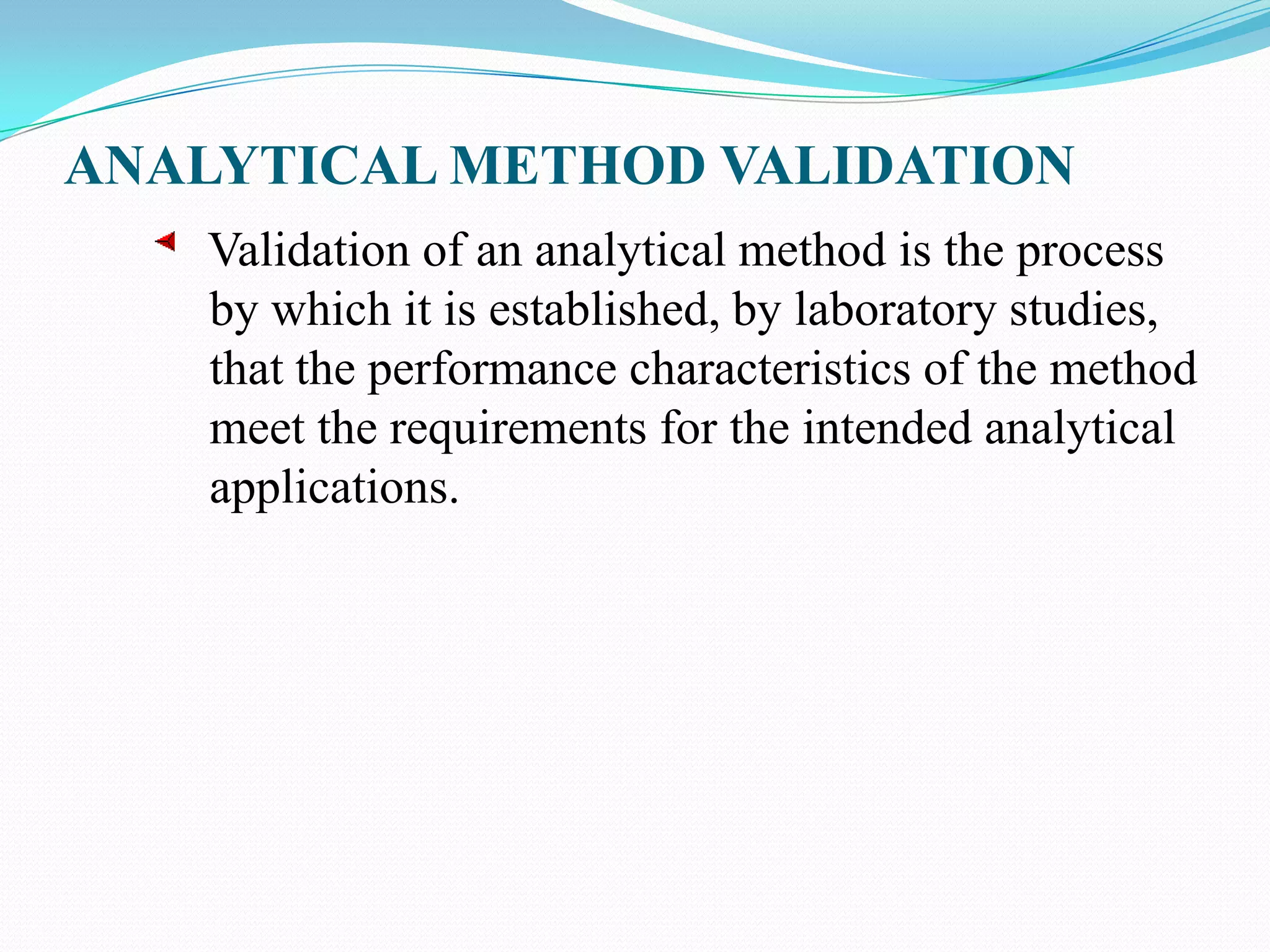
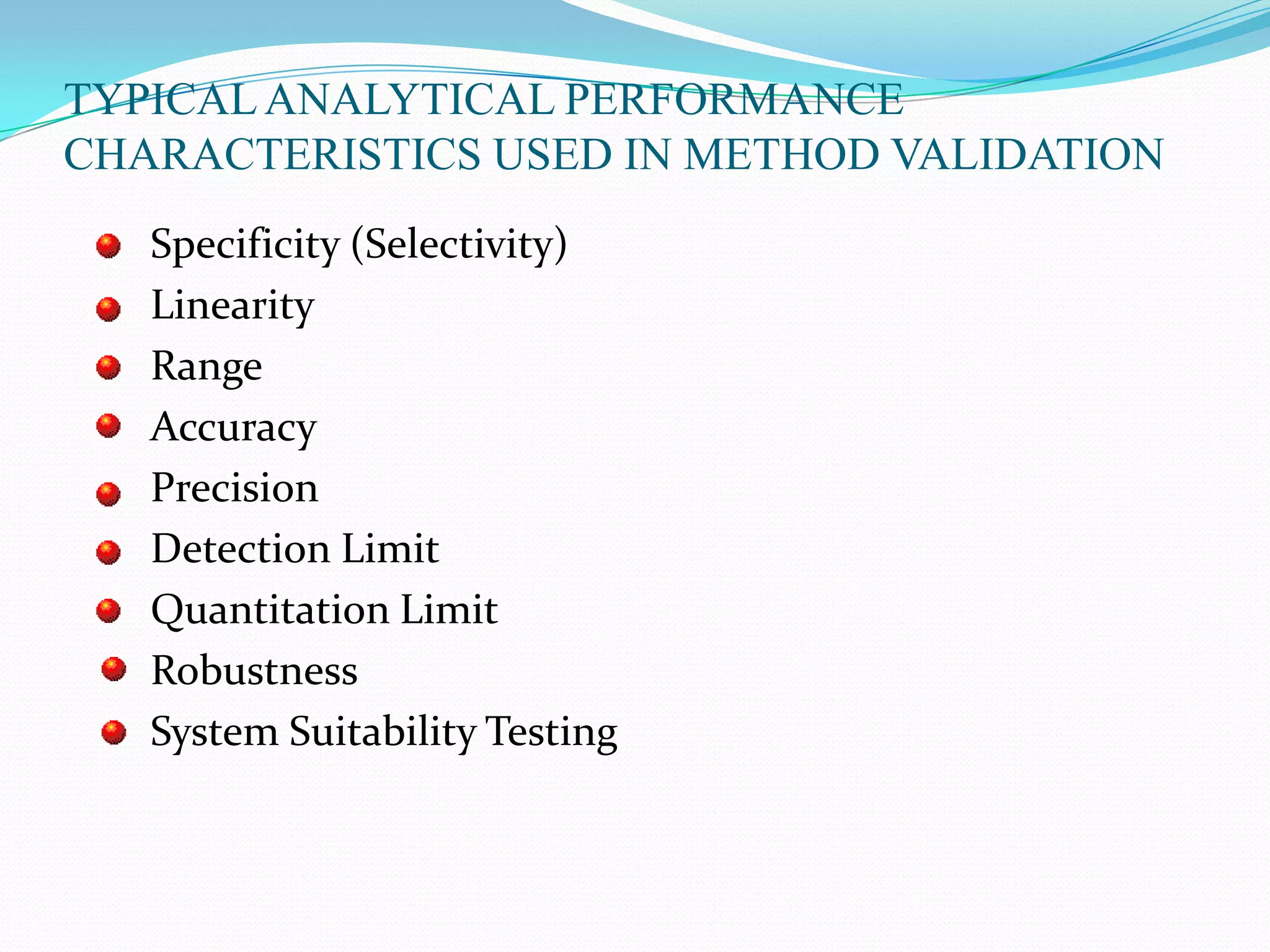
This document discusses guidelines for analytical method validation. It outlines types of analytical methods that require validation including chromatographic, spectroscopic, and dissolution methods. Key analytical performance characteristics used in validation are described such as specificity, linearity, range, accuracy, precision, detection/quantitation limits, robustness, and system suitability testing. The document provides details on determining these characteristics and validating methods. It also addresses revalidation and references for further information.


















![The United State Pharmacopoeia 24; The National
Formulary 19; 2000: [1225] VALIDATION OF
COMPENDIAL METHODS.
www.labcompliance.com/methods/meth_va
htm#introduction
http://www.fda.gov/cder/guidance/2396dft.htm
www.fda.gov/ohrms/dockets/
ac/02/slides/3841s1_07_lachman.PPT
http://www.fda.gov/cder/guidance/ameth.htm](https://image.slidesharecdn.com/analyticalmethodvalidation-121127224712-phpapp01/75/Analytical-method-validation-24-2048.jpg)

