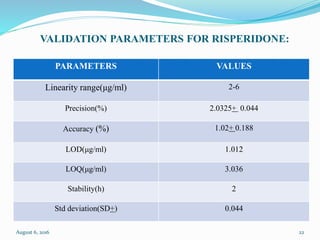
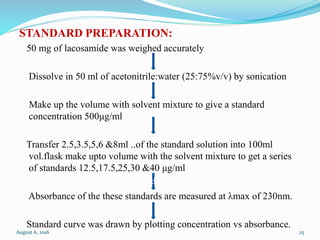
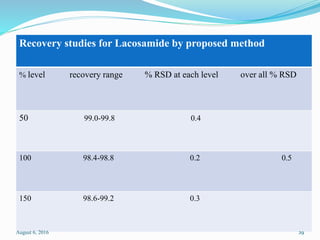
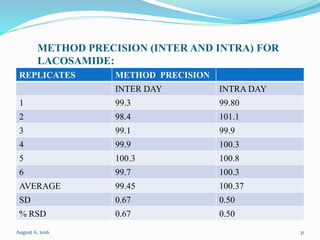
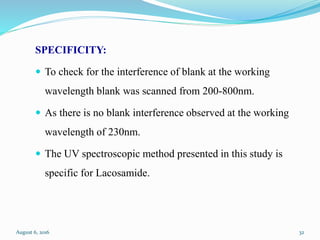
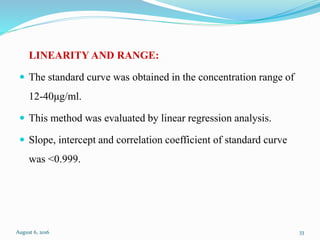
The document describes the development and validation of UV spectrophotometric methods for analyzing risperidone and lacosamide. It discusses selecting analytical wavelengths, developing standard curves, and validating the methods by determining accuracy, precision, specificity, linearity, range and other parameters as required by ICH guidelines. Validation results for the risperidone and lacosamide methods such as recovery percentages between 98.4-99.8%, precision of 0.67-0.50%, linear ranges of 2-6 μg/ml and 12-40 μg/ml respectively are also presented. The developed and validated methods provide accurate and precise quantification of active pharmaceutical ingredients and finished dosage forms using UV spectrophotometry.