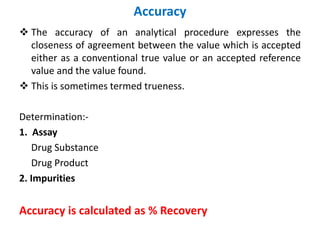
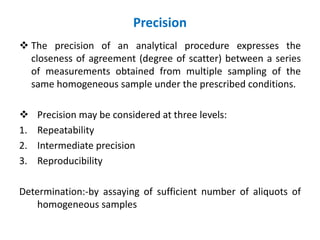
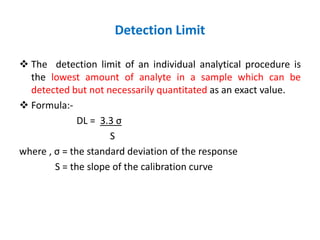
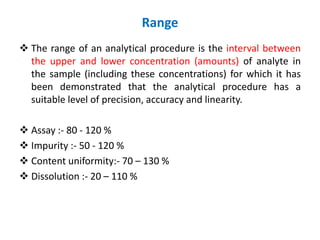
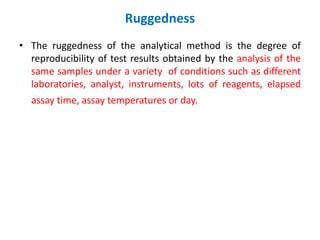
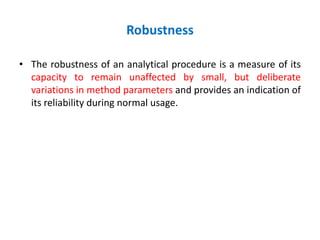
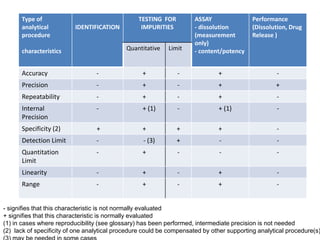
This document discusses analytical method validation as per ICH and USP guidelines. It defines validation as establishing documentary evidence that a procedure maintains compliance. Method validation involves demonstrating that an analytical procedure is suitable for its intended purpose by testing parameters such as accuracy, precision, specificity, detection limit, quantitation limit, linearity, range, ruggedness and robustness. It also discusses the different types of analytical procedures that require validation including identification tests, quantitative impurity tests, limit tests and assays.