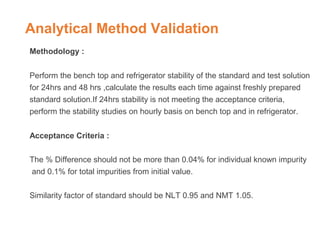
This document provides an overview of analytical method validation. It defines validation as demonstrating a method is suitable for its intended purpose. Key validation characteristics discussed include precision, accuracy, specificity, linearity, range, detection limit, quantitation limit, ruggedness and robustness. The document describes the methodology for evaluating each characteristic, such as spiking known concentrations of analytes and establishing acceptance criteria. It emphasizes that validation confirms a method consistently produces results meeting pre-defined standards of quality.