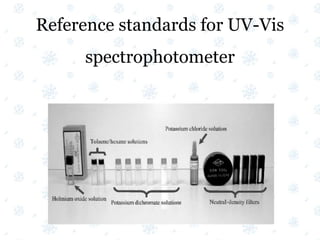
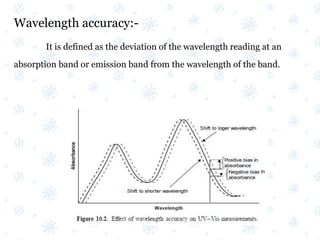
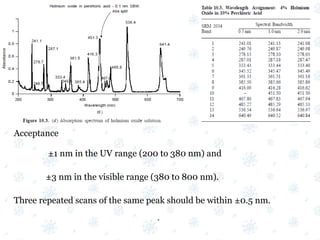
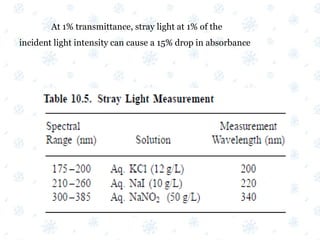
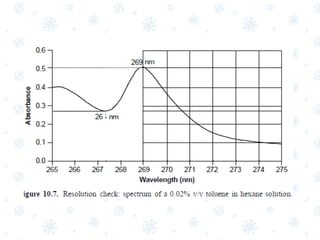
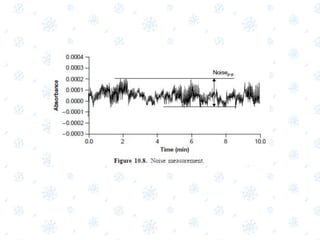
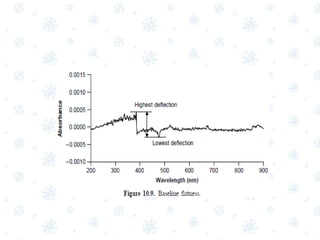
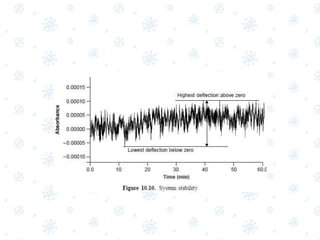
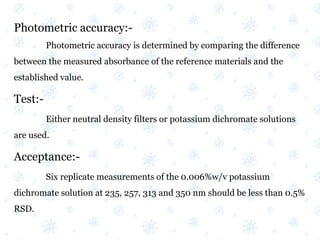
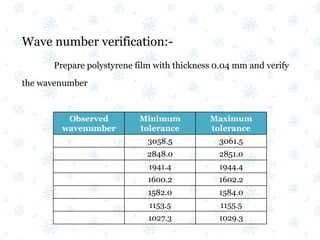
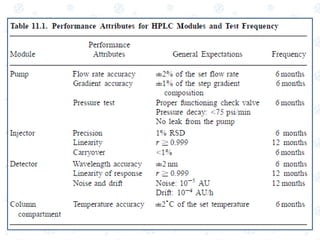
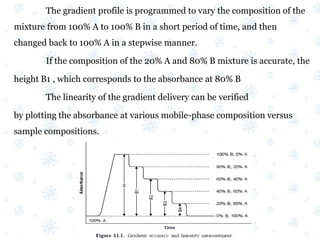
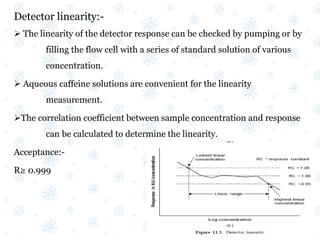
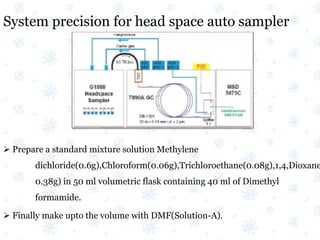
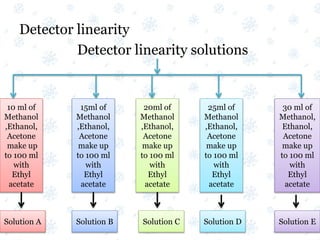
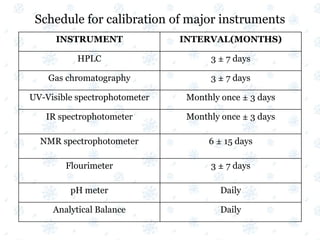
This document discusses the calibration and validation of various analytical instruments used in pharmaceutical analysis. It provides details on calibrating UV-Vis spectrophotometers, IR spectrophotometers, spectrofluorimeters, HPLC, and GC. Calibration ensures instrument readings are accurate against standards, while validation confirms the instrument is correctly installed and operating as intended. The document outlines tests and acceptance criteria for evaluating characteristics like wavelength accuracy, resolution, noise, baseline flatness, sensitivity, flow rate, and linearity during calibration and validation of different analytical instruments.