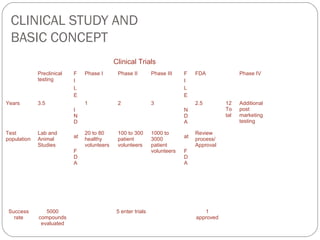
Clinical trials progress through phases (preclinical, I-IV) to evaluate treatments safely in humans. Preclinical testing occurs in labs and animals. Phase I studies evaluate safety in 20-80 healthy volunteers. Phase II expands to 100-300 patient volunteers to assess efficacy. Phase III further tests efficacy in 1,000-3,000 patients. FDA approval requires compliance with Good Clinical Practice guidelines to protect subject rights and ensure credible data. Key elements include oversight by independent review boards, informed consent, qualified investigators and sponsors, adherence to protocols, and comprehensive record keeping.