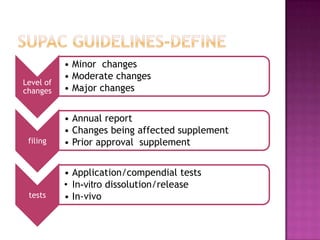
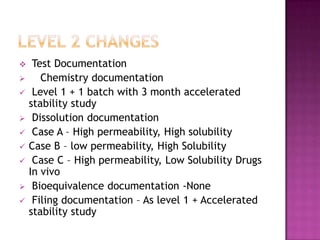
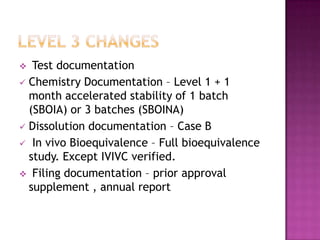
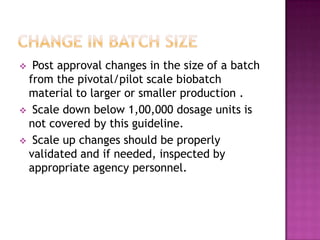
The document discusses guidelines for post-approval changes to drug products, including changes to batch size, manufacturing sites and equipment, and composition. It outlines 3 levels of changes - minor, moderate, and major - and provides recommendations for documentation and regulatory filings required for each level of change. Major changes, such as a new manufacturing site or changes in the amount of active ingredients, require more extensive documentation including stability testing and possibly bioequivalence studies.