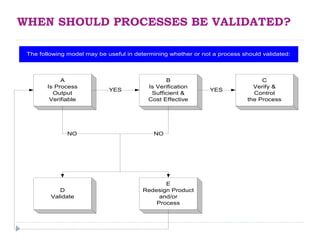
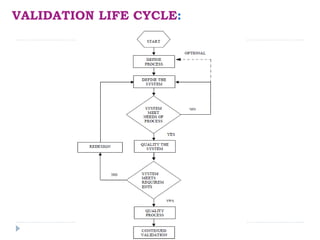
This document discusses process validation in the pharmaceutical industry. It defines process validation and describes it as having three stages: process design, process qualification, and continued process verification. The objectives and requirements of each stage are explained. Process validation helps ensure a process consistently produces products meeting specifications and quality attributes. It involves understanding and controlling sources of variation. Validation protocols, reports, teams, and the lifecycle are also reviewed to explain how process validation is planned and documented.