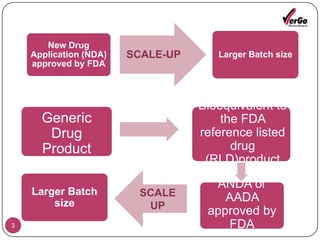
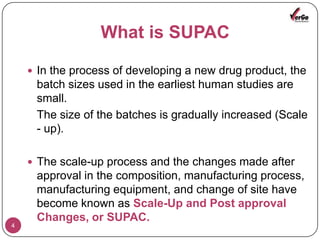
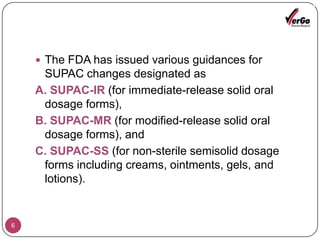
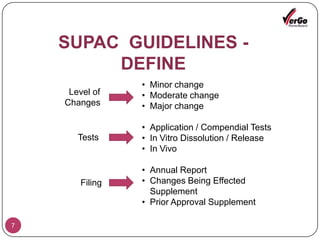
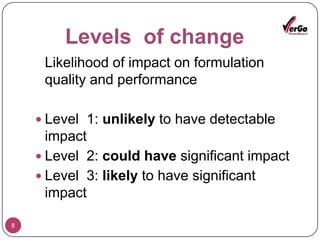
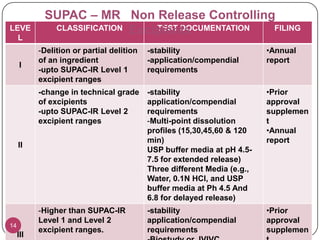
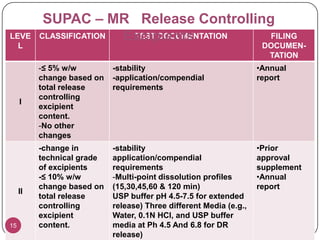
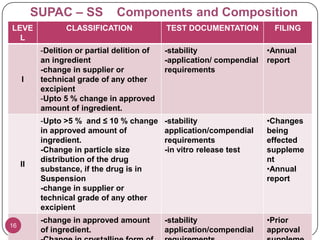
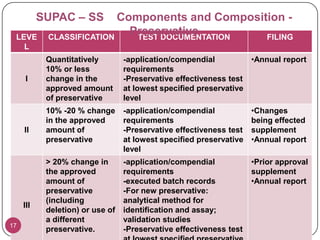
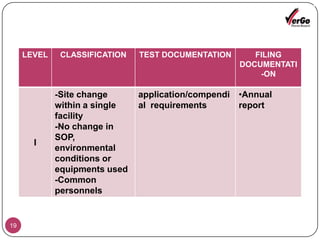
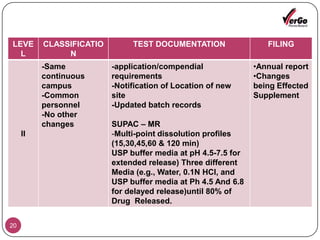
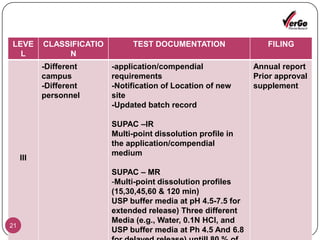
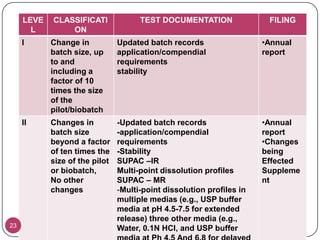
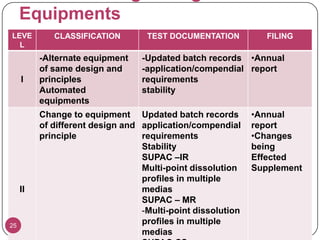
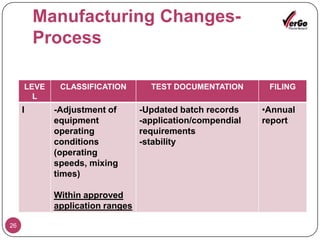
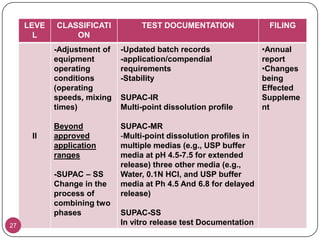
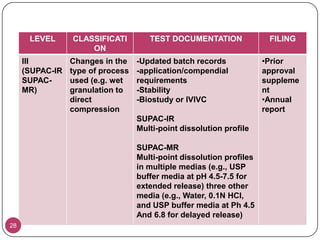
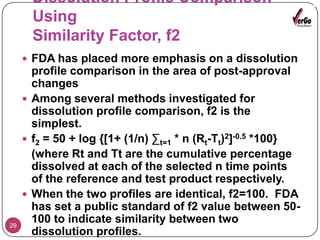
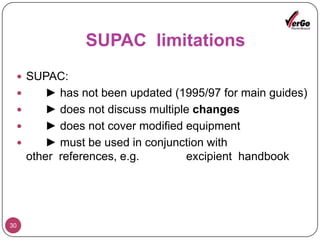
The document discusses the Scale-Up and Post Approval Changes (SUPAC) guidelines issued by the FDA for generic drug products, detailing changes in components, manufacturing processes, and batch sizes. It outlines three main categories of SUPAC changes for immediate-release, modified-release, and non-sterile semisolid dosage forms, emphasizing the need for validation and compliance with current good manufacturing practices. Additionally, it highlights the importance of dissolution profile comparisons and notes limitations such as outdated guidelines and the need for supplementary references.