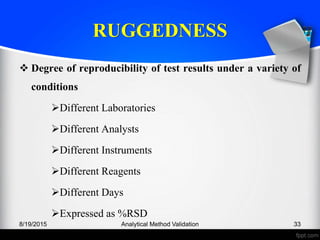
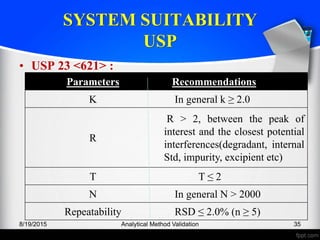
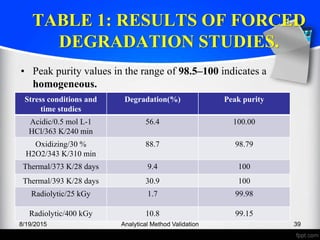
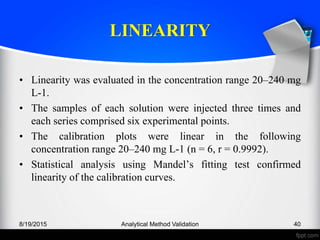
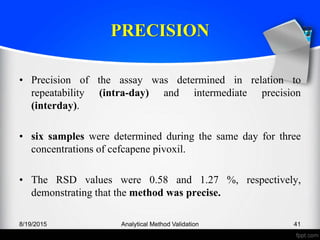
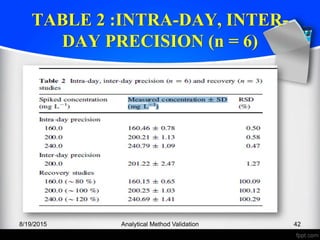
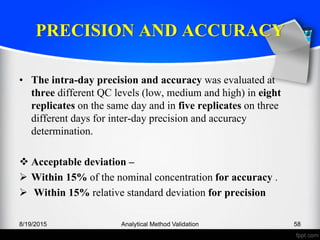
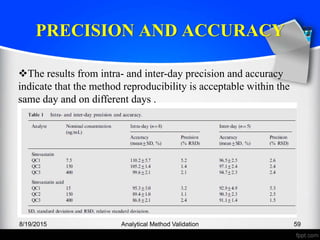
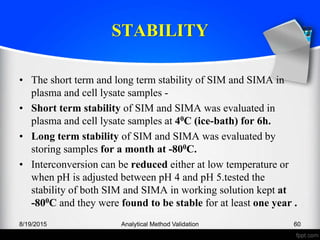
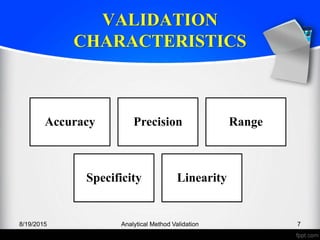
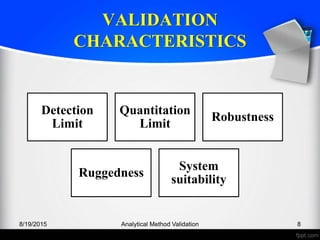
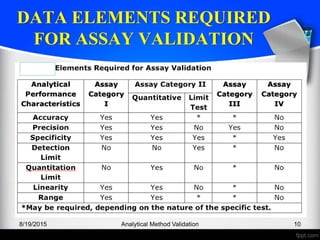
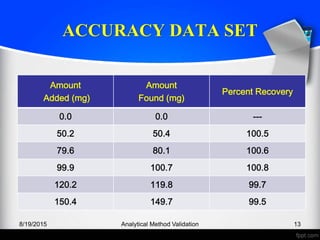
The document outlines the regulatory guidelines and considerations for analytical method validation, focusing on parameters such as accuracy, precision, specificity, and robustness, based on various established standards. It details criteria for different types of analytical procedures, validation characteristics, and the importance of systematic evaluations like system suitability. Additionally, the document presents examples of validation studies conducted on specific compounds to demonstrate the application of these principles.











![DETECTION LIMIT
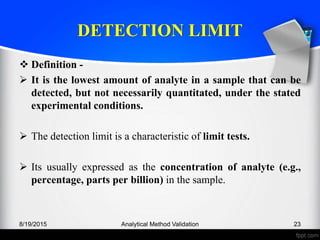
Determination -
For non instrumental procedures -
• Generally determined by the analysis of samples with known
concentrations of analyte and by establishing the minimum
level at which the analyte can be reliably detected.
For instrumental procedures -
• That exhibit background noise, which is to compare measured
signals from samples with known low concentrations of
analyte with those of blank samples.
• [Acceptable signal-to-noise ratios are 2:1 or 3:1.]
8/19/2015 Analytical Method Validation 24](https://image.slidesharecdn.com/analyticalmethodvalidation-150819092607-lva1-app6892/85/Analytical-method-validation-by-manoj-ingale-best-ppts-24-320.jpg)

![QUANTITATION LIMIT
Determination -
For non instrumental procedures -
• Determined by the analysis of samples with known
concentrations of analyte
For instrumental procedures -
• the ICH documents describe a common approach, which is to
compare measured signals from samples with known low
concentrations of analyte with those of blank samples.
[A typically acceptable signal-to-noise ratio is 10:1.]
8/19/2015 Analytical Method Validation 26](https://image.slidesharecdn.com/analyticalmethodvalidation-150819092607-lva1-app6892/85/Analytical-method-validation-by-manoj-ingale-best-ppts-26-320.jpg)