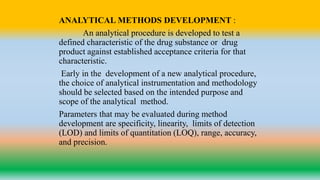
This document provides a summary of analytical method validation. It discusses the types of analytical procedures that should be validated, including identification tests, quantitative impurity tests, limit tests, and active moiety assays. It also summarizes key validation characteristics that should be considered, such as accuracy, precision, specificity, range, detection limit, quantitation limit, linearity, and robustness. The document provides definitions and methodology recommendations for validating analytical procedures. It emphasizes that the validation process verifies that an analytical method is suitable for its intended purpose.