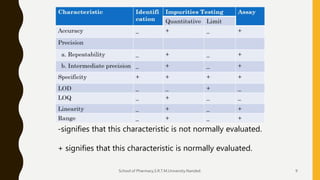
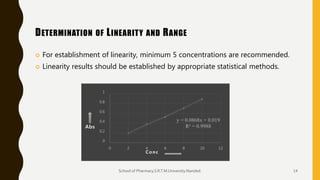
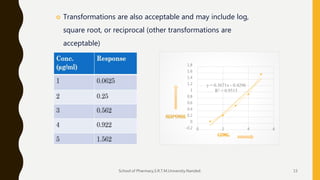
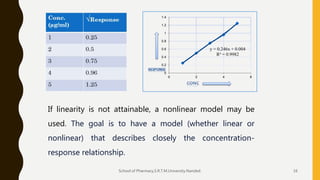
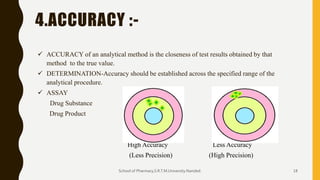
The document discusses validation of analytical procedures. It defines validation as establishing by laboratory studies that an analytical procedure meets requirements for its intended use. It describes the typical steps in validating identification tests, quantitative impurity tests, and assays. Key validation characteristics discussed include specificity, linearity, range, accuracy, precision, detection and quantitation limits, robustness, and ruggedness. The guidelines provide details on establishing each characteristic to help ensure analytical methods are suitable for their intended pharmaceutical applications.