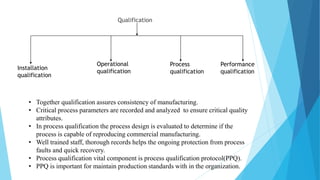
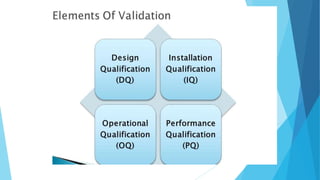
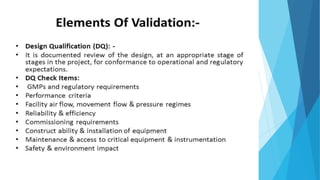
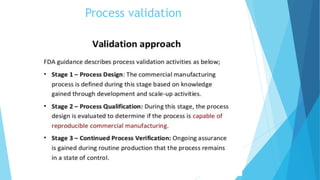
This document outlines the importance of qualification and validation in pharmaceutical technology transfer, highlighting three major stages of process validation as specified by the US FDA. It emphasizes the need for well-documented protocols and trained personnel to ensure manufacturing consistency, quality assurance, and compliance with regulations. Validation is presented as an ongoing process that not only enhances drug quality but also leads to economic benefits through reduced testing and sampling procedures.